DL-Laudanosine
- CAS NO.:1699-51-0
- Empirical Formula: C21H27NO4
- Molecular Weight: 357.44
- MDL number: MFCD00006910
- EINECS: 216-923-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
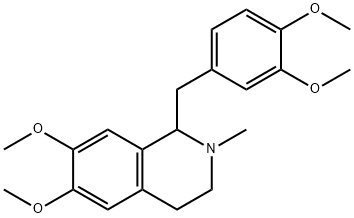
What is DL-Laudanosine?
Description
This opium alkaloid occurs in the liquor following precipitation of Thebaine (q.v.) and is purified by extraction with small quantities of Et20, followed by precipitation with potassium iodide. The base crystallizes from hot C6H6 in small, colourless needles and has [α]15D+ 103.23° (EtOH). It is freely soluble in CHCI3, EtOH, Et20 or hot C6H6 but insoluble in H20 or alkalies. No colour is produced with FeCl 3 but with Fe203 and H2S04, a brown colour is formed, changing to green when warmed to ISOoC. With concentrated H2S04 alone, the alkaloid gives a rose-red colour, changing to deep violet at ISO°C. The solution of the alkaloid in EtOH is alkaline to litmus and both it, and its salts, are bitter to the taste. The crystalline methiodide has m.p. 218-221°C; [α]D + 120°. The oxidation products with Mn02 and H2S04 are veratraldehyde, 2:3:6:7- tetramethoxy-9: I O-dihydroanthracene and laudaline (4: S-dimethoxy-2: - methylaminoethylbenzaldehyde, m.p. 123-4°C. On exhaustive methylation it yields trimethylamine and laudanosene (tetramethoxy-o-vinylstilbene). Laudanosine is one of the most convulsant of the opium alkaloids but possesses only a slight analgesic action.
Chemical properties
light yellow fine crystalline powder
The Uses of DL-Laudanosine
DL-Laudanosine is an intermediate in synthesizing (S)-Laudanosine (L178525), a metabolite of the neuromuscular-blocking drugs Atracurium (A794500) and Cisatracurium (C496700) with potentially toxic systemic effects. It crosses the blood-brain barrier and may cause excitement and seizure activity.
Definition
ChEBI: 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinoline is a member of isoquinolines.
Purification Methods
Crystallise these from EtOH. The (±)-picrate crystallises from EtOH with m 177-178o. The (-)-isomer has m 83-85o and
References
Pictet, Athanasescu., Ber., 33, 2347 (1900)
Pictet, Finkelstein., ibid, 42, 1979 (1909)
Pyman, Reynolds.,J. Chern. Soc., 97, 1324 (1910)
Kondo, Mori.,J. Ph arm. Soc., Japan, 51,615 (1931)
Craig, Tarbell., J. Amer. Chern. Soc., 70,2783 (1948)
Synthesis:
Pictet, Finkelstein., Compt. rend., 148,925 (1909)
Elliot.,J. Heterocycl. Chern., 7, 1229 (1970)
Pharmacology:
Zunz., Elements de Pharmacodynamie speciale, Tome I, 178, Masson & Co.,
Paris (1932)
Krueger, Eddy, Sumwalt., U.S. Public Health Reports, Suppl. 165, 1007
(1943)
Properties of DL-Laudanosine
| Melting point: | 115°C |
| Boiling point: | 490.07°C (rough estimate) |
| Density | 1.1729 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 100 mg/mL (279.77 mM) |
| pka | 7.80±0.40(Predicted) |
| form | Solid |
| color | White to Light Beige |
Safety information for DL-Laudanosine
Computed Descriptors for DL-Laudanosine
DL-Laudanosine manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Dl-laudanosine 95% CAS 1699-51-0View Details
Dl-laudanosine 95% CAS 1699-51-0View Details
1699-51-0 -
 Laudanosine CAS 1699-51-0View Details
Laudanosine CAS 1699-51-0View Details
1699-51-0 -
 ATRACURIUM BESYLATE - IMPURITY G 90 % AboveView Details
ATRACURIUM BESYLATE - IMPURITY G 90 % AboveView Details
1699-51-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
