Trypan Blue
Synonym(s):Trypan Blue;Direct blue 14;Direct Blue 14, AzidinBlue;Niagara blue reagent;Blue cell viability dye
- CAS NO.:72-57-1
- Empirical Formula: C34H24N6Na4O14S4
- Molecular Weight: 960.81
- MDL number: MFCD00003969
- EINECS: 200-786-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
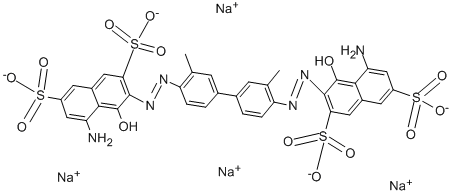
What is Trypan Blue?
Chemical properties
Trypan Blue is a dark Blue crystalline solid or powder that dissolves in water to form a blue solution. It's slightly soluble in soluble fiber yarn and doesn't dissolve in other organic solvents. When mixed with concentrated sulfuric acid, it turns dark greenish-blue, and when diluted, it becomes reddish-blue. With concentrated nitric acid, it creates a brownish-gray solution. In a 10% sulfuric acid solution, its aqueous solution turns red, while with sodium hydroxide, it becomes reddish-purple.
The Uses of Trypan Blue
Biological stain.
The Uses of Trypan Blue
Trypan Blue is an azo based, hydrophilic, tetrasulfonated blue acid dye widely utilized for assessing cell viability. As an easy method to determining the viability of cells, Trypan Blue will stain dead cells with permeable membranes blue, while the dye is excluded by most living cells and their intact membranes thereby allowing visual determination of living versus dead cells. Although Trypan Blue is predominately used for assessing cell viability of cultured cells, other applications for this dye have been reported. In rat abdominal organ slices Trypan Blue has been utilized to determine cell viability as well as islet cell cluster cell viability from caprine pancreatic samples. Dyes and metabolites.
The Uses of Trypan Blue
Trypan blue has been used as a dye in trypan blue exclusion assay/cell viability assay to detect dead cells.
What are the applications of Application
Trypan Blue is a viability dye for quantifying live versus dead cells
Definition
ChEBI: Trypan blue is an organosulfonate salt that is the tetrasodium salt of 3,3'-[(3,3'-dimethylbiphenyl-4,4'-diyl)didiazene-2,1-diyl]bis(5-amino-4-hydroxynaphthalene-2,7-disulfonic acid). It has a role as a histological dye, a fluorochrome and a carcinogenic agent. It is an organosulfonate salt and an organic sodium salt. It contains a trypan blue(4-).
Preparation
3,3′-Dimethylbenzidine double nitriding, in alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid(2 Moore) coupled.
General Description
Bluish-gray to dark blue powder.
Air & Water Reactions
Azo dyes can be explosive when suspended in air at specific concentrations. Slightly soluble in water.
Reactivity Profile
Direct Blue 14 is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. Direct Blue 14 is incompatible with strong oxidizing agents and strong acids.
Health Hazard
ACUTE/CHRONIC HAZARDS: Direct Blue 14 may cause irritation and may be absorbed through the skin. It is a positive animal carcinogen. When heated to decomposition Direct Blue 14 emits very toxic fumes of nitrogen oxides, sulfur oxides, carbon monoxide and carbon dioxide.
Fire Hazard
Flash point data for Direct Blue 14 are not available; however, Direct Blue 14 is probably combustible.
Biochem/physiol Actions
Trypan Blue is excluded by most living cells, but can be taken into phagocytes and certain other cells.
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by intraperitoneal, intravenous, and subcutaneous routes. Experimental teratogenic and reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx NazO, and SOx
Potential Exposure
Used in dyeing textiles; leather and paper; as a biological stain.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3143 dyes, solid, toxic, n.o.s. or dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Properties and Applications
blue. Blue gray powder. Soluble in water for blue, slightly soluble in soluble fiber element, insoluble in other organic solvents. The strong sulfuric acid in dark green light for blue, diluted into a red light blue; In the light of nitric acid for palm gray solution. The dye solution to join a red sulfuric acid 10% change; Add thick sodium hydroxide solution for red light purple.
| Standard | Acid Resistance | Alkali Resistance | Light Fastness | Soaping | Water | ||
| Fading | Stain | Fading | Stain | ||||
| ISO | 4-5 | 4 | 1-2 | 2 | 2 | ||
| AATCC | 5 | 2 | 1-2 | 2 | 1 | ||
Incompatibilities
Dust may form explosive mixture with air. Trypan Blue is incompatible with strong oxidizing agents. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Azo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acylhalides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combinations can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. Trypan Blue is sensitive to prolonged exposure to heat.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Trypan Blue
| Melting point: | >300 °C (lit.) |
| Density | 1.007 g/mL at 20 °C |
| storage temp. | dry at room temperature |
| solubility | H2O: soluble10mg/mL |
| form | Powder |
| Colour Index | 23850 |
| color | Dark greenish-brown |
| PH | 9.8 (10g/l, H2O, 20℃) |
| Water Solubility | 10 g/L (25 ºC) |
| Merck | 14,9792 |
| BRN | 4360496 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 72-57-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 8, Sup 7) 1987 |
| EPA Substance Registry System | Trypan blue (72-57-1) |
Safety information for Trypan Blue
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Trypan Blue
Trypan Blue manufacturer
Appex Dye Stuff Industries
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 72-57-1 98%View Details
72-57-1 98%View Details
72-57-1 -
 Trypan Blue CAS 72-57-1View Details
Trypan Blue CAS 72-57-1View Details
72-57-1 -
 Trypan Blue CAS 72-57-1View Details
Trypan Blue CAS 72-57-1View Details
72-57-1 -
 Trypan Blue CASView Details
Trypan Blue CASView Details -
 Trypan blue CAS 72-57-1View Details
Trypan blue CAS 72-57-1View Details
72-57-1 -
 Direct Blue 14 CAS 72-57-1View Details
Direct Blue 14 CAS 72-57-1View Details
72-57-1 -
 Trypan Blue Solution CAS 72-57-1View Details
Trypan Blue Solution CAS 72-57-1View Details
72-57-1 -
 DIRECT BLUE 14 (C.I. 23850) CASView Details
DIRECT BLUE 14 (C.I. 23850) CASView Details