DIPHACINONE
- CAS NO.:82-66-6
- Empirical Formula: C23H16O3
- Molecular Weight: 340.37
- MDL number: MFCD00046317
- EINECS: 201-434-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
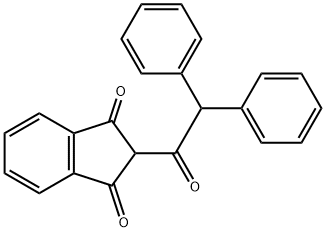
What is DIPHACINONE?
Description
Diphacinone is also called 2-(diphenylacetyl)indan-1,3-dione, is a yellow powder which is practically insoluble in water, readily soluble in chloroform, toluene, xylene, acetone, ethanol, heptane, alkalis [9, p. 431].
Originator
Dipaxin,Upjohn,US,1955
The Uses of DIPHACINONE
Diphacinone is an anticoagulant rodenticide widely used to control rodent infestations.
The Uses of DIPHACINONE
Rodenticide.
The Uses of DIPHACINONE
Diphacinone is used for the control of rats, mice, voles, prairie dogs, ground squirrels and other rodents.
What are the applications of Application
Diphacinone is an anticoagulant rodenticide used to control infestations
Production Methods
Diphacinone is produced by condensation of 1,1-diphenyl acetone with dimethyl phthalate in the presence of sodium methoxide (30).
Definition
ChEBI: Diphenadione is a diarylmethane and a beta-triketone.
Manufacturing Process
A solution of sodium methoxide was prepared by adding 2.76 grams (0.12mol) of sodium to 50 ml of absolute methanol and gently warming the
mixture to effect complete solution of the sodium. To this was added 300
milliliters of dry benzene with vigorous stirring, whereafter excess methanol
was removed by concentrating the mixture to a volume of about 100 ml. To
the resulting sodium methoxide suspension was added a solution of 19.4
grams (0.1 mol) of dimethyl phthalate in 200 ml of dry benzene. The mixture
was heated to boiling and a solution of 21 grams (0.1 mol) of diphenylacetone
in 200 ml of dry benzene was added dropwise thereto. During addition
approximately 200 ml of liquid, which consisted of benzene together with
methanol formed during the course of the reaction, was distilled from the
reaction mixture. After addition of the diphenylacetone, the mixture was
heated under reflux for about 6 hours, cooled and stirred vigorously with 200
ml of 5% sodium hydroxide solution.
The light yellow solid which separated was collected by filtration; the filtrate
was reserved for treatment as described below. Suspension in water of the
solid, which weighed 12 grams, and acidification of the mixture with dilute
hydrochloric acid produced a gum which soon crystallized. Recrystallization of
this solid from ethanol gave 10.2 grams (30%) of 2-diphenylacetyl-1,3-
indandione as a light yellow crystalline solid, which melted at 146-147°C.
The filtrate mentioned above consisted of 3 layers. An oily layer which was
present between the aqueous and benzene layers was separated, acidified and
extracted with ether. The aqueous layer was likewise separated, acidified and
extracted with ether. The extracts were combined, dried and evaporated to
yield a heavy gum which was crystallized from ethanol to give an additional
2.5 grams of product which melted at 146-147°C. The total yield of 2-
diphenylacetyl-1,3-indandione was 12.7 grams (37%).
Therapeutic Function
Anticoagulant
General Description
Odorless pale yellow crystals. Used as a rodenticide and anticoagulant medication.
Air & Water Reactions
Practically insoluble in water (17mg/L). Hydrolyzed by strong acid.
Reactivity Profile
DIPHACINONE is a ketone, and behaves as a weak acid. Forms water soluble alkali metal salts. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
DIPHACINONE is extremely toxic; probable oral lethal dose in humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150-lb. person. Many medical conditions will be aggravated by DIPHACINONE.
Fire Hazard
When heated to decomposition DIPHACINONE emits acrid smoke and fumes. Sensitive to light.
Agricultural Uses
Rodenticide: A U.S. EPA restricted Use Pesticide (RUP) when the formulation contains 3% or more of diphacinone. Diphacinone is an anti-coagulant rodenticide bait used for control of rats, mice, voles and other rodents. It is available in meal, pellet, wax block, and liquid bait formulations, as well as in tracking powder and concentrate formulations. It is used in general agriculture and in food-processing areas. The top five uses for diphacinone in California are on landscapes, general vertebrate pest control, around structures and right of ways, and on oranges. This material is also used as an anticoagulant medication. Not approved for use in EU countries.
Trade name
DE-PESTER®[C]; DIDANDIN®; DIPAXIN®; DITRAC®; GOLD CREST®; KILL-RO RAT KILLER®; LIQUA-TOX®, diphacinone sodium salt; ORAGULANT®; P. C. Q. ®; PID®; PROMAR®; RAMIK®; RAT KILLER®; RODENT CAKE®[C]; SOLVAN®; TOMCAT®; U 1363®
Safety Profile
Poison by ingestion. Inlxbits blood clotting, leading to hemorrhages. Action similar to coumadin (warfarin). A pesticide used in rodent control. When heated to decomposition it emits acrid smoke and irritating fumes
Potential Exposure
This material is used as a rodenticideand as an anticoagulant medication.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attenti decontamination and life support for the victims.Emergency personnel should wear protective clothingappropriate to the type and degree of contamination. Airpurifying or supplied-air respiratory equipment should alsobe worn, as necessary. Rescue vehicles should carry supplies, such as plastic sheeting and disposable plastic bags,to assist in preventing spread of contaminationon immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Obtain authorization and/or further instructions from thelocal hospital for administration of an antidote or performance of other invasive procedures. Rush to a health-carefacility. Acute exposure to Diphacinone may require
Metabolic pathway
Diphacinone is a member of the indandione class of anti-coagulants. Its fate in rats and mice has been reported but no information on its degradation in soil or plants has been published. It is metabolised by hydroxylation and conjugation.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area
Shipping
Coumarin-derivative pesticides, solid, toxic,require a shipping label of “POISONOUS/TOXICMATERIALS.” It falls in Hazard Class 6.1 and diphacinonein Packing Group I
Degradation
Diphacinone is stable in solution at pH 6-9 for 14 days; it is hydrolysed in less than 24 hours at pH 4 (PM). It is rapidly degraded under conditions of aqueous photolysis (PM).
Incompatibilities
This material is extremely toxic;probable oral lethal dose in humans is 550 mg/kg, orbetween 7 drops and 1 teaspoonful for a 150-lb person.Diphacinone is an anticoagulant (inhibits blood clotting).Hemorrhage is the most common effect and may be manifested by nose bleeding, gum bleeding, bloody stools andurine, ecchymoses (extravasations of blood into skin), andhemoptysis (coughing up blood). Bruising is heightened.Abdominal and flank pains are also common. Other signsand symptoms include flushing, dizziness, hypotension (lowblood pressure), dyspnea (shortness of breath), cyanosis (bluetint to the skin and mucous membranes), fever, and diarrhea
Properties of DIPHACINONE
| Melting point: | 146-147° |
| Boiling point: | 436.18°C (rough estimate) |
| Density | 1.1454 (rough estimate) |
| vapor pressure | 1.37 x l0-8 Pa (25 °C) |
| refractive index | 1.6700 (estimate) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 2.79±0.10(Predicted) |
| Water Solubility | 0.3 mg l-1 |
| form | Solid |
| color | Light Yellow to Yellow |
| CAS DataBase Reference | 82-66-6(CAS DataBase Reference) |
| EPA Substance Registry System | Diphacinone (82-66-6) |
Safety information for DIPHACINONE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P350:IF ON SKIN: Gently wash with plenty of soap and water. |
Computed Descriptors for DIPHACINONE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
