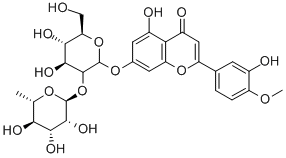
Diosmetin
Synonym(s):3′,5,7-Trihydroxy-4′-methoxyflavone;4′-Methylluteolin;5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one;Luteolin 4′-methyl ether
- CAS NO.:520-34-3
- Empirical Formula: C16H12O6
- Molecular Weight: 300.26
- MDL number: MFCD00017425
- EINECS: 208-291-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
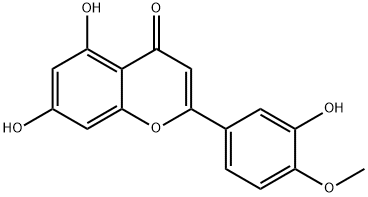
What is Diosmetin?
Chemical properties
Diosmetin is a flavonoid compound that appears as a yellow powder. It is extracted from lemon and also found in various other natural plants, including spearmint and spider incense. Diosmetin is slightly soluble in methanol but readily dissolves in DMSO.
The Uses of Diosmetin
Diosmetin (Diosmin EP Impurity F) is a metabolite of Apigenin. Antibacterial.
What are the applications of Application
Diosmetin is a flavonoid that impedes human CYP1A enzyme activity and has anti-mutagenic and anti-allergenic properties
Definition
ChEBI: Diosmetin is a monomethoxyflavone that is the 4'-methyl ether derivative of luteolin. It is a natural product isolated from citrus fruits which exhibits a range of pharmacological activities. It has a role as an antioxidant, an antineoplastic agent, a plant metabolite, a tropomyosin-related kinase B receptor agonist, an apoptosis inducer, an angiogenesis inhibitor, a cardioprotective agent, a bone density conservation agent, an anti-inflammatory agent and a vasodilator agent. It is a monomethoxyflavone, a trihydroxyflavone and a 3'-hydroxyflavonoid. It is functionally related to a luteolin. It is a conjugate acid of a diosmetin-7-olate.
Flammability and Explosibility
Not classified
Biological Activity
diosmetin (dio) is an agonist of the aryl hydrocarbon receptor (ahr). it potently inhibited the enzyme activity of cytochrome p450 1a1 (cyp1a1) in a dose-dependent manner with an ic50 value of approximately 30 nm, in microsomes from mcf-7 cells [1].ahr belongs to the per, arnt, sim/basic-helix-loop-helix superfamily of ligand-activated transcription factors. ahr mediates the toxic effects of polycyclic aromatic hydrocarbons, 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) and polychlorinated biphenyls. these chemicals all bind to ahr, and result in the activation of a battery of genes, including the cytochromes p450 cyp1a1, cyp1a2, and cyp1b1 [2].in mcf-7 cells, at 24 h after the incubation of diosmetin, cyp1a1 mrna was increased in a dose-dependent manner. in mcf-7 cells, diosmetin at 2.5 μm modestly increased cyp1a1 enzyme activity, with an activity increase in cells, while diosmetin at 5 μm did not increase the enzyme activity compared to controls in cells. compared with controls, diosmetin dose-dependently increased the capacity of nuclear extracts to bind an oligonucleotide containing the ahr-binding sequence of cyp1a1 [1].in the presence of cyp1a inhibitor, the concentration of diosmetin ranged from 25 μm at 0 h to 22 μm. in the absence of cyp1a inhibitor, the concentration of diosmetin ranged from 25 μm at 0 h to 15 μm [3].no in vivo result from the administration of diosmetin had been found.
References
[1]. ciolino hp, wang tt and yeh gc. diosmin and diosmetin are agonists of the aryl hydrocarbon receptor that differentially affect cytochrome p450 1a1 activity. cancer res, 1998, 58(13):2754-60. PMID: 9661887
[2]. gonzalez fj and fernandez-salguero p. the aryl hydrocarbon rreceptor studies using the ahr-null mice. drug metabolism and disposition, 1998, 26(12): 1194-1198. PMID: 9860927
[3]. and routsopoulos vp and spandidos da. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. j nutr biochem, 2013, 24(2):496-504. DOI:10.1016/j.jnutbio.2012.01.012
Properties of Diosmetin
| Melting point: | 256-258°C |
| Boiling point: | 576.7±50.0 °C(Predicted) |
| Density | 1.512±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: soluble1mg/mL, clear, light yellow to yellow |
| form | Solid |
| form | neat |
| pka | 6.50±0.40(Predicted) |
| color | light yellow to yellow |
| Water Solubility | 19μg/L at 20℃ |
| BRN | 294492 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 520-34-3(CAS DataBase Reference) |
Safety information for Diosmetin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Diosmetin
| InChIKey | MBNGWHIJMBWFHU-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Diosmetin, 99% CAS 520-34-3View Details
Diosmetin, 99% CAS 520-34-3View Details
520-34-3 -
 Diosmetin, 98% CAS 520-34-3View Details
Diosmetin, 98% CAS 520-34-3View Details
520-34-3 -
 Diosmetin 98% (HPLC) CAS 520-34-3View Details
Diosmetin 98% (HPLC) CAS 520-34-3View Details
520-34-3 -
 Diosmetin CAS 520-34-3View Details
Diosmetin CAS 520-34-3View Details
520-34-3 -
 Diosmetin CAS 520-34-3View Details
Diosmetin CAS 520-34-3View Details
520-34-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
