Dichlorophen
Synonym(s):Bis(5-chloro-2-hydroxyphenyl)methane;Dichlorophen;Dichlorophene
- CAS NO.:97-23-4
- Empirical Formula: C13H10Cl2O2
- Molecular Weight: 269.12
- MDL number: MFCD00002322
- EINECS: 202-567-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
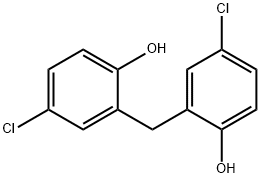
What is Dichlorophen?
Chemical properties
white or off-white powder
Originator
Dichlorophen,Aquapharm
The Uses of Dichlorophen
Dichlorophen exhibits algicidal, bactericidal and fungicidal activities. It is a contact fungicide used to control dollar spots and rots in turf. It is also an anthelminthic used for the treatment of various tapeworms in human and domestic animals.
The Uses of Dichlorophen
anthelmintic
The Uses of Dichlorophen
Agricultural fungicide; antimicrobial; germicide in soaps, shampoos, etc.
What are the applications of Application
Dichlorophen is an effective fungicide against celluloytic fungi
Definition
ChEBI: Dichlorophen is a diarylmethane and a bridged diphenyl fungicide.
Manufacturing Process
2.520 g of sulfuric acid (93%) is stirred and cooled to 0°C. A solution of 552 g of p-chlorophenol in 305 g of methyl alcohol is run into the acid, the
temperature being kept below 10°C. The mixture is cooled to -5°C and a
solution of 170 g of aqueous formaldehyde solution (37% CH2O in water) in
332 g of methyl alcohol is introduced at a more or less uniform rate over a
period of 4 hours. The temperature of the reaction mixture is not allowed to
rise above 0°C. After all of the formaldehyde-containing solution has been
added, the batch is stirred for 3 hours longer at a temperature of -5°-0°C.
Enough ice is then added to the contents of the reaction chamber in order to
reduce the sulfuric acid concentration to 70%. 2,2'-Dihydroxy-5,5'-dichlorodiphenyl methane is extracted from the resulting mixture with a mixture of
1.069 g of isopropyl ether and 1.575 g of toluene. Ice is added until the acid
concentration is about 30%. The acid layer is removed and the solvent layer is
washed acid-free. Most of the isopropyl ether is removed by atmospheric
distillation with a fractionating column, the temperature of the escaping
vapors not being permitted to exceed 90°C. From the residue, about 280 g of
pure 2,2'-dihydroxy-5,5'-dichloro-diphenyl methane, MP: 177°-178°C,
crystallize. The product is filtered, washed with toluene and dried at about
100°C. By concentrating the mother liquor remaining after the foregoing
crystallization and filtration, another 225 grams of substantially pure 2,2'-
dihydroxy-5,5'-dichloro-diphenyl methane are obtained. This latter crop may
be crystallized from toluene in order to convert it into 2,2'-dihydroxy-5,5'-
dichlorodiphenyl methane of melting point of 177°-178°C.
Therapeutic Function
Antiseptic, Anthelmintic, Antifungal
General Description
White slightly cream or light pink-colored powder. Melting point 177°C. Slight phenolic odor and a saline phenolic taste. Moderately toxic. Used as a fungicide and bactericide.
Air & Water Reactions
Slowly oxidized in air. Insoluble in water.
Reactivity Profile
Dichlorophen is incompatible with strong oxidizing agents and strong bases . Weakly acidic.
Fire Hazard
Flash point data for Dichlorophen are not available; however, Dichlorophen is probably combustible.
Agricultural Uses
Fungicide, Herbicide, Bactericide, Veterinary medicine: Not currently registered in the U.S. Dichlorophene is a wide-spectrum, non-oxidizing biocide used against all types of algae and bacteria. Widely used to treat fungi, fleas and worm conditions in pet animals and livestock. See U.S. Food and Drug Administration 20 CFR 520.580 and 20 CFR 520.581
Trade name
ANTHIPHEN®; DIPHENTANE 70®; DICHLOROPHEN®; DICHLOROPHEN B®; DICHLOROPHENE 10®; DICHLORPHEN®; DIDROXANE®; DIPHENTHANE 70®; FUNGICIDE F®; FUNGICIDE GM®; FUNGICIDE M®; G 4®; GEFIR®; HYOSAN; KORIUM®; PLATH-LYSE®; PREVENTAL®; PREVENTOL®; PREVENTOL GD®; PREVENTOL GDC®; SUPER MOSSTOX®; TAENIATOL®; TENIATOL®; TENIATHANE®; TRIVEX®; VERMITHANA®; WESPURIL®
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. A skin and severe eye irritant. Mutation data reported. Can cause cramps and diarrhea. Possibly similar to DDT. An FDA over-the counter drug. An anthelmintic. When heated to decomposition it emits toxic fumes of Cl-.
Metabolic pathway
Limited information is available to describe the degradation and metabolic fate of dichlorophen. A photodegradation study showed that dichlorophen undergoes hydroxydechlorination and dechlorination reactions as the major degradation pathways. Direct conjugation of one or both hydroxyl groups with sulfate and/or glucuronic acid was observed as the major metabolic pathway in the rat.
Purification Methods
Crystallise dichlorophen from toluene. [Beilstein 6 III 5406.]
Degradation
Dichlorophen (1) underwent hydroxydechlorination in acidic solution (pH 5.6) when irradiated under a xenon lamp (280 and 300 nm). 4-Chloro- 4'-hydroxy-2,2'-methylenediphenol(2) was the major product from reactions conducted in the absence of oxygen. A benzoquinone-like tautomer (3) was detected in oxygenated solution. A dechlorination product [4- chloro-2,2'-methylenediphenol(4)] was also observed as a minor product (Mansfield and Richard, 1996). These pathways are shown in Scheme 1.
Properties of Dichlorophen
| Melting point: | 168-172 °C (lit.) |
| Boiling point: | 418.7±40.0 °C(Predicted) |
| Density | 1.3239 (estimate) |
| vapor pressure | 1.3 x 10-5 Pa (25 °C) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in methanol, ether |
| pka | pK1:7.6;pK2:11.5 (25°C) |
| form | Solid |
| form | neat |
| color | White to Light yellow to Light orange |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| Merck | 14,3071 |
| BRN | 1884514 |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 97-23-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol], 2,2'-methylenebis[4-chloro-(97-23-4) |
| EPA Substance Registry System | Dichlorophene (97-23-4) |
Safety information for Dichlorophen
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dichlorophen
Dichlorophen manufacturer
Earthchem Laboratories P Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
![97-23-4 Dichlorophen [ BP] 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/ce21aec5-3daa-4b66-8237-f0a612ac453b.png) 97-23-4 Dichlorophen [ BP] 98%View Details
97-23-4 Dichlorophen [ BP] 98%View Details
97-23-4 -
 97-23-4 98%View Details
97-23-4 98%View Details
97-23-4 -
 2,2'-Methylenebis(4-chlorophenol) CAS 97-23-4View Details
2,2'-Methylenebis(4-chlorophenol) CAS 97-23-4View Details
97-23-4 -
 Dichlorophen 97-23-4 98%View Details
Dichlorophen 97-23-4 98%View Details
97-23-4 -
 97-23-4 Dichlorophen 98%View Details
97-23-4 Dichlorophen 98%View Details
97-23-4 -
 Dichlorophen 99%View Details
Dichlorophen 99%View Details
97-23-4 -
 Di Chlorophene CASView Details
Di Chlorophene CASView Details -
 Bis(5-chloro-2-hydroxyphenyl)methane CAS 97-23-4View Details
Bis(5-chloro-2-hydroxyphenyl)methane CAS 97-23-4View Details
97-23-4