Dexlansoprazole Impurity 7
- CAS NO.:1781244-56-1
- Empirical Formula: C23H16F3N5OS
- Molecular Weight: 467.47
- Update Date: 2022-12-21 16:56:50
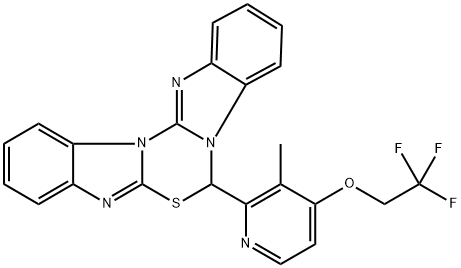
What is Dexlansoprazole Impurity 7?
The Uses of Dexlansoprazole Impurity 7
7-(3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)-7H-benzo[4,5]imidazo[2,1-b]benzo[4,5]imidazo[2,1-d][1,3,5]thiadiazine is a basic degradation product of the drug Lansoprazole (L175000), a gastric proton pump inhibitor and an antiulcerative.
Properties of Dexlansoprazole Impurity 7
| Boiling point: | 666.6±65.0 °C(Predicted) |
| Density | 1.55±0.1 g/cm3(Predicted) |
| pka | 3.82±0.37(Predicted) |
Safety information for Dexlansoprazole Impurity 7
Computed Descriptors for Dexlansoprazole Impurity 7
Abamectin manufacturer
GLP Pharma Standards
2Y
Phone:+91-9866074638
Whatsapp: +91 9866074638
product: 1781244-56-1 Dexlansoprazole Degradation Impurity 98%
New Products
SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Azithromycin Imp D Azithromycin EP Impurity C Atracurium Besylate EP Impurity D Azithromycin EP Impurity B Azithromycin EP Impurity O Cetirizine Glycerol Ester Impurity HydrochlorideYou may like
-
 1781244-56-1 Dexlansoprazole Degradation Impurity 98%View Details
1781244-56-1 Dexlansoprazole Degradation Impurity 98%View Details
1781244-56-1 -
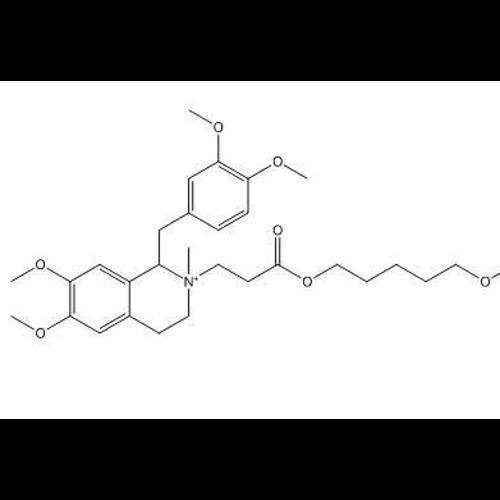 Atracurium Besylate EP Impurity CView Details
Atracurium Besylate EP Impurity CView Details
86293-35-8 -
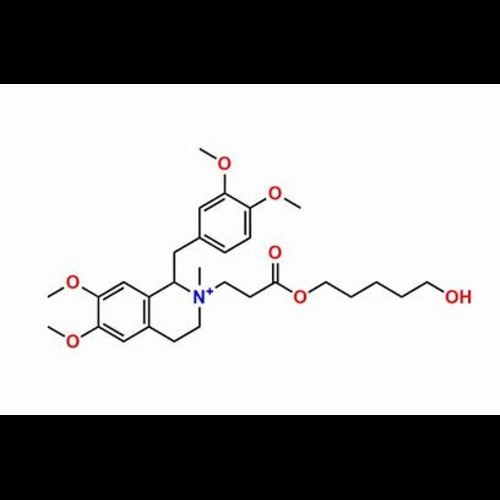 Atracurium Besylate EP Impurity DView Details
Atracurium Besylate EP Impurity DView Details
87046-72-8 -
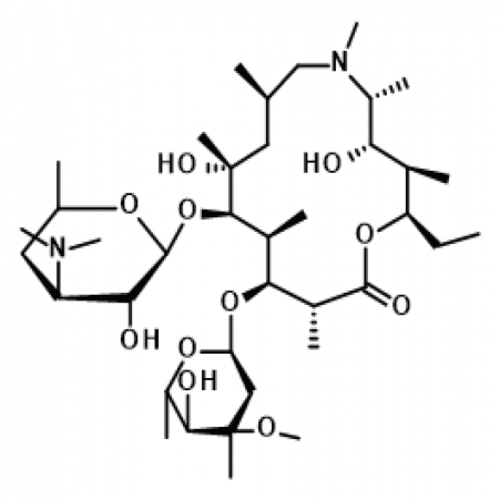 Azithromycin EP Impurity BView Details
Azithromycin EP Impurity BView Details
307974-61-4 -
 Azithromycin EP Impurity CView Details
Azithromycin EP Impurity CView Details
620169-47-3 -
 Azithromycin EP Impurity OView Details
Azithromycin EP Impurity OView Details
763924-54-5 -
 Azithromycin Imp DView Details
Azithromycin Imp DView Details
612069-26-8 -
 Ceftaroline fosamil Acetate HydrateView Details
Ceftaroline fosamil Acetate HydrateView Details
400827-55-6
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.