Dapagliflozin propanediol monohydrate
- CAS NO.:960404-48-2
- Empirical Formula: C24H33ClO8
- Molecular Weight: 484.97
- MDL number: MFCD28167768
- EINECS: 811-335-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
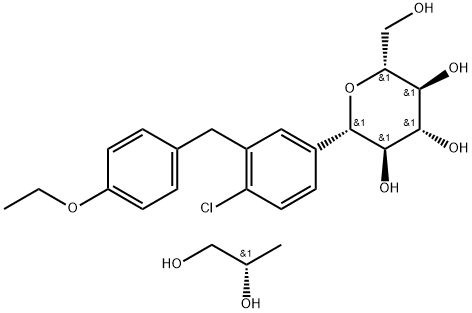
What is Dapagliflozin propanediol monohydrate?
Description
Dapagliflozin propanediol monohydrate is the first approved sodium-glucose cotransporter protein 2 (SGLT2) inhibitor. It is indicated for the treatment of type 2 diabetes. When combined with diet and exercise in adults, dagliflozin helps improve glycaemic control by inhibiting glucose reabsorption in the proximal tubules of renal units and causing glycosuria. Dagliflozin can be given alone or in combination with insulin or other oral hypoglycaemic agents as adjunctive therapy.
Dagliflozin was originally approved by the FDA on 8 January 2014 for use in combination with diet and exercise to improve glycemic control in adults with type 2 diabetes. It was later approved in April 2021 to reduce the risk of declining kidney function, kidney failure, cardiovascular death and hospitalisation for heart failure in adults with chronic kidney disease.
The Uses of Dapagliflozin propanediol monohydrate
Dapagliflozin Propanediol Hydrate is an SGLT2 inhibitor, which can be used for the treatment of diabetes.
Definition
ChEBI: A hydrate that consists of dapagliflozin compounded with (S)-propylene glycol and hydrate in a (1:1:1) ratio. Used to improve glycemic control, along with diet and exercise, in adults with type 2 diabetes.
Clinical Use
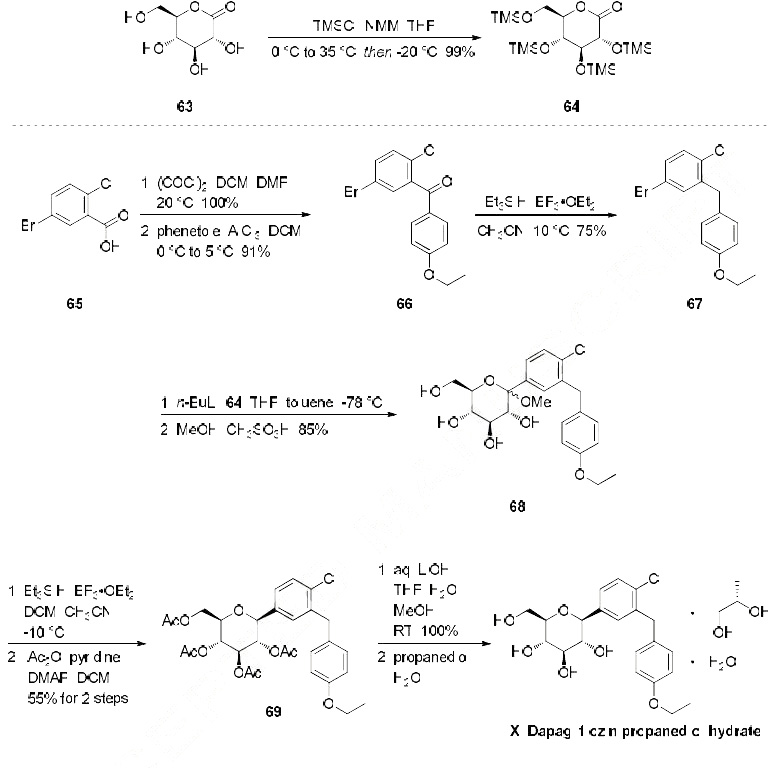
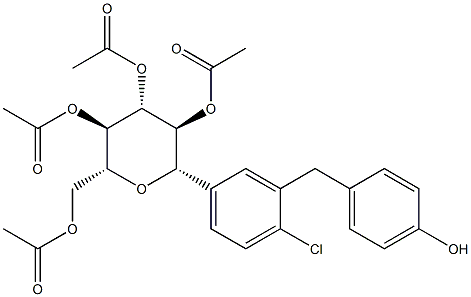
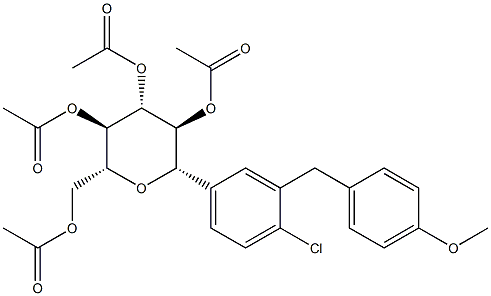
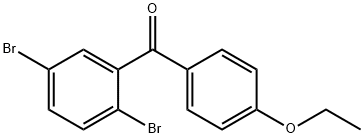
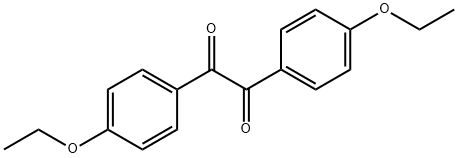
The most likely process-scale synthesis has been described in a literature publication and patent, and this is summarized in the scheme below. The synthesis began with global silylation glucolactone 63 to form tetrasiloxide 64. In parallel, commercial 5-bromo-2-chlorobenzoyl acid (65) was converted to the corresponding acid chloride with oxalyl chloride. Subsequently, this acid chloride was subjected to Friedel-Crafts acylation with ethyl phenyl ether (“phenetole”) in the presence of aluminum trichloride at low temperature to give benzophenone 66 in 91% yield. Next, the carbonyl functionality within 66 was removed upon treatment with triethylsilane and boron trifluoride-etherate, producing 5-bromo-2-chloro- 4'-ethoxydiphenylmethane 67 in 75% yield as the aglycon partner. Aryl bromide 67 was subjected to lithium halogen exchange conditions and subsequent exposure to lactone 64, which gave a mixture of lactols which were then immediately subjected to methanesulfonic acid to provide glucol 68 in 85% yield. The anomeric methoxy group of 68 was reduced with triethylsilane and boron trifluoride-etherate followed by peracetylation to deliver α-C-glycoside tetra-acetate 69 in 55% (two steps) after recrystalliaztion in ethanol. Hydrolysis of polyacetate 69 with lithium hydroxide gave dapagliflozin in quantitative yield, and upon treatment with propanediol in water, dapagliflozin propanediol hydrate (X) was produced.
Synthesis
Dapagliflozin propanediol hydrate, an orally active sodium glucose cotransporter type 2 (SGLT-2) inhibitor, was developed by Bristol-Myers Squibb (BMS) and AstraZeneca for the once-daily treatment of type 2 diabetes. As opposed to competitor SGLT-2 inhibitors, dapagliflozin was not associated with renal toxicity or long-term deterioration of renal function in phase III clinical trials. The drug exhibits excellent SGLT2 potency with more than 1200 fold selectivity over the SGLT1 enzyme.
Properties of Dapagliflozin propanediol monohydrate
| Melting point: | 74 - 78°C |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for Dapagliflozin propanediol monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dapagliflozin propanediol monohydrate
Dapagliflozin propanediol monohydrate manufacturer
Piramal Pharma Solutions
Honour Lab Limited
ZCL Chemicals Ltd
Vanquest Pharma Pvt Ltd
Kopran Ltd
Emmennar Pharma Pvt Ltd
BDR Pharmaceuticals International Pvt Ltd
Cohance Lifesciences (Previously RA Chem Pharma Ltd)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran



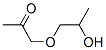
You may like
-
 Dapagliflozin propanediol monohydrate 99%View Details
Dapagliflozin propanediol monohydrate 99%View Details
960404-48-2 -
 960404-48-2 Dapagliflozin propanediol monohydrate 98%View Details
960404-48-2 Dapagliflozin propanediol monohydrate 98%View Details
960404-48-2 -
 Dapagliflozin propanediol monohydrate 960404-48-2 98%View Details
Dapagliflozin propanediol monohydrate 960404-48-2 98%View Details
960404-48-2 -
 960404-48-2 99%View Details
960404-48-2 99%View Details
960404-48-2 -
 Dapagliflozin propanediol monohydrate 98%View Details
Dapagliflozin propanediol monohydrate 98%View Details
960404-48-2 -
 960404-48-2 98%View Details
960404-48-2 98%View Details
960404-48-2 -
 960404-48-2 Dapagliflozin propanediol monohydrate 98%View Details
960404-48-2 Dapagliflozin propanediol monohydrate 98%View Details
960404-48-2 -
 Dapagliflozin propanediol monohydrate CAS 960404-48-2View Details
Dapagliflozin propanediol monohydrate CAS 960404-48-2View Details
960404-48-2