Daclatasvir Dihydrochloride
- CAS NO.:1009119-65-6
- Empirical Formula: C40H51ClN8O6
- Molecular Weight: 775.35
- MDL number: MFCD25541736
- EINECS: 816-965-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 19:43:17
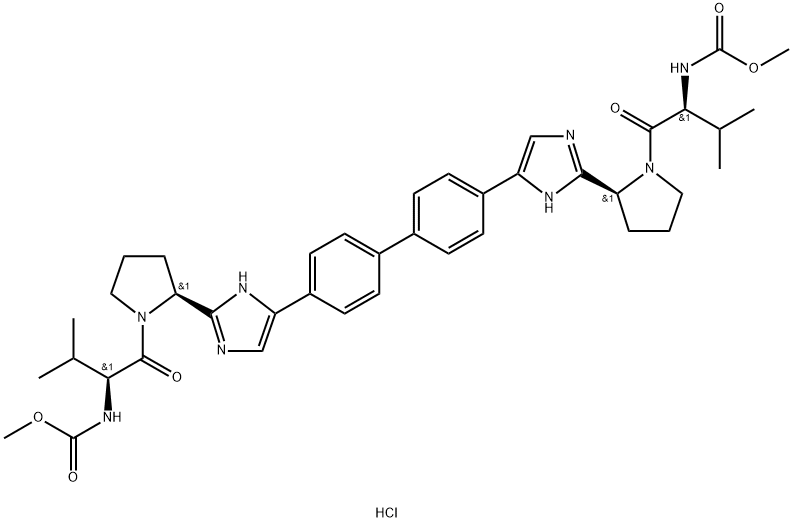
What is Daclatasvir Dihydrochloride?
Description
Daclatasvir dihydrochloride is a hepatitis C virus nonstructural 5A (NS5A) replication complex inhibitor which was first approved in Japan for the treatment of genotype 1 HCV patients who fail to respond to interferon plus ribavirin. The drug has also been approved for patients with untreated, chronic HCV who are eligible for interferon. Additionally, in Europe, daclatasvir was approved for use in combination with other products across genotype 1–4 HCV. Daclatasvir was discovered and developed by Bristol–Myers Squibb and a fascinating account describing the initiation of the program from a phenotypic screen and the medicinal chemistry strategy leading to the discovery of the compound has been recently reported.
Chemical properties
Daclatasvir dihydrochloride is white to yellow powder. It is freely soluble in water (>700 mg/mL). The solubility is strongly pH-dependent and the solubility is high at low pH values.
The Uses of Daclatasvir Dihydrochloride
Daclatasvir Dihydrochloride is the dihydrochloride salt form of daclatasvir, an orally available inhibitor of the hepatitis C virus (HCV) non-structural protein 5A (NS5A) replication complex, with potential activity against HCV. Daclatasvir is used with another antiviral medication (sofosbuvir) to treat chronic (long-lasting) hepatitis C, a viral infection of the liver. Daclatasvir should never be used without sofosbuvir. Daclatasvir and sofosbuvir may also be used with another antiviral medication (ribavirin).
Definition
ChEBI: Daclatasvir hydrochloride is a hydrochloride obtained by combining daclatasvir with two molar equivalents of hydrochloric acid. It is a potent inhibitor of nonstructural protein 5A and is used for treatment of hepatitis C. It has a role as an antiviral drug and a nonstructural protein 5A inhibitor. It contains a daclatasvir(2+).
Synthesis
Bromination of commercial 4,40-diacetylbiphenyl (58) gave 4,40-bis(bromoacetyl)biphenyl 59 in 82% yield. Alkylation of NBoc- L-proline (60) with 59 gave diester 61 which was treated with ammonium acetate to effect cyclization of the bis-ketoester to provide bis-imidazole 62 in 63% yield for the two steps. Acidic removal of the Boc protecting groups followed by recrystallization provided bis-pyrrolidine 63 in high yield. Acylation of 63 with N-(methoxycarbonyl)- L-valine (64) using N-(3-dimethylaminopropyl)-N0-ethylcarbodiimide (EDC) and 1-hydroxybenxotriazole hydrate (HOBT) provided declatasvir. The dihydrochloride salt was prepared and treated with Cuno Zet Carbon ? followed by crystallization from acetone to give daclatasvir dihydrochloride (IX) in 74% yield.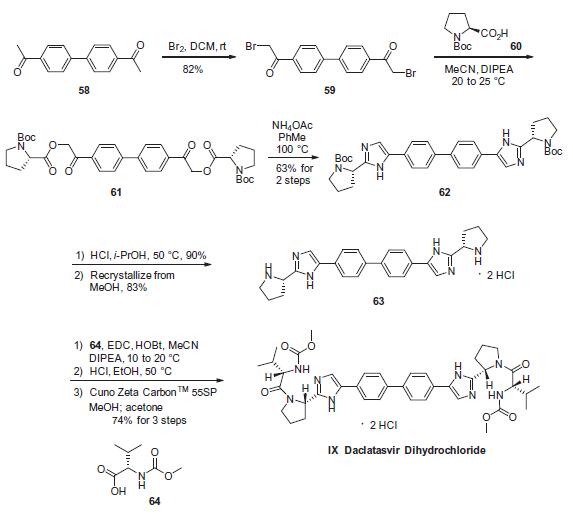
Side Effects
Tiredness, headache, nausea, or diarrhea may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Mode of action
Daclatasvir is an inhibitor of nonstructural protein 5A (NS5A), a multifunctional protein that is an essential component of the HCV replication complex. Daclatasvir binds to the N-terminus of NS5A and inhibits both viral RNA replication and virion assembly.
Properties of Daclatasvir Dihydrochloride
| Melting point: | >207°C (dec.) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | Off-White to Yellow |
Safety information for Daclatasvir Dihydrochloride
Computed Descriptors for Daclatasvir Dihydrochloride
Daclatasvir Dihydrochloride manufacturer
Glenmark Pharmaceuticals Limited
Arene Lifesciences Limited
SETV ASRV LLP
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1,1'-[1,1'-biphenyl]-4,4'-diylbis[2,2-dibromo]-ethanone](https://img.chemicalbook.in/CAS/20180601/GIF/28179-17-1.gif)
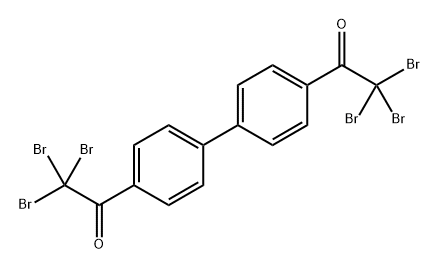
![1-{4'-acetyl-[1,1'-biphenyl]-4-yl}-2-bromoethan-1-one](https://img.chemicalbook.in/CAS/20180702/GIF/36934-45-9.gif)
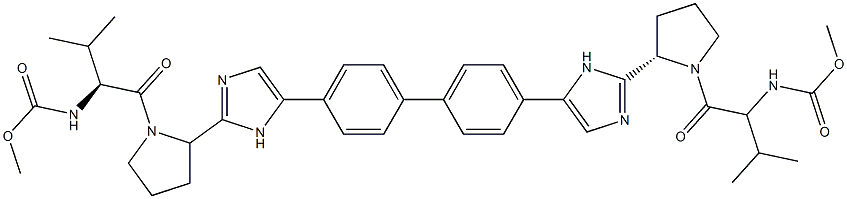
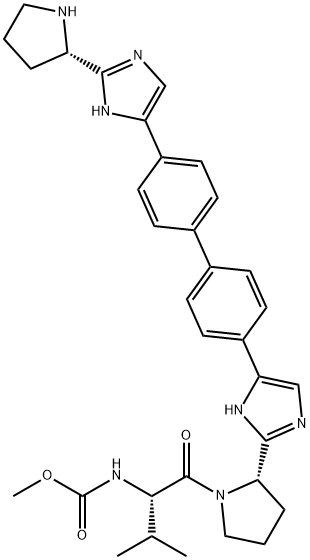
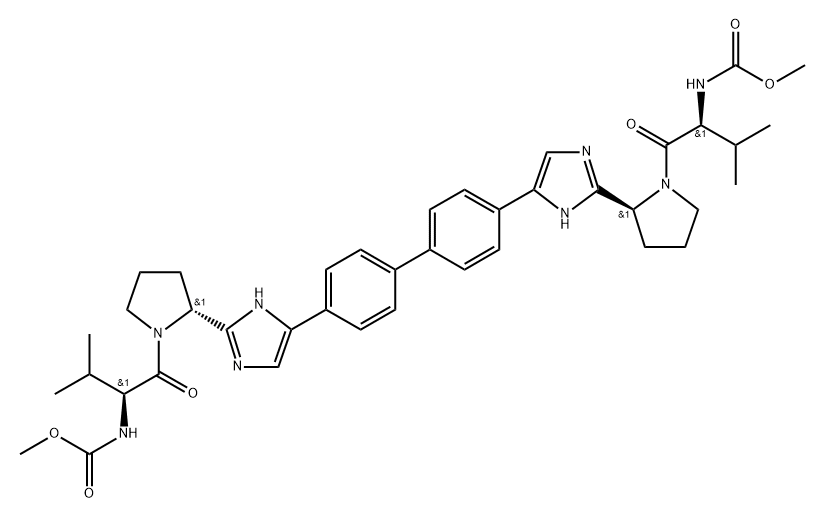
![methyl ((S)-1-((S)-2-(5-(4'-(2-((S)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-yl)-1H-imidazol-4-yl)-[1,1'-biphenyl]-4-yl)-1H-imidazol-2-yl)pyrrolidin-1-yl)-3-methyl-1-oxobutan-2-yl)carbamate](https://img.chemicalbook.in/CAS/20180527/GIF/1007884-53-8.gif)

You may like
-
 1009119-65-6 98%View Details
1009119-65-6 98%View Details
1009119-65-6 -
 Daclatasvir Hcl In-House 98%View Details
Daclatasvir Hcl In-House 98%View Details
1009119-65-6 -
 Daclatasvir Hcl 99%View Details
Daclatasvir Hcl 99%View Details -
 Daclatasvir dihydrochloride 1009119-65-6 98%View Details
Daclatasvir dihydrochloride 1009119-65-6 98%View Details
1009119-65-6 -
 1009119-65-6 98%View Details
1009119-65-6 98%View Details
1009119-65-6 -
 1009119-65-6 Daclatasvir dihydrochloride 98%View Details
1009119-65-6 Daclatasvir dihydrochloride 98%View Details
1009119-65-6 -
 Daclatasvir dihydrochloride 95% CAS 1009119-65-6View Details
Daclatasvir dihydrochloride 95% CAS 1009119-65-6View Details
1009119-65-6 -
 1009119-65-6 DACLATASVIR DI HCL 95-99 %View Details
1009119-65-6 DACLATASVIR DI HCL 95-99 %View Details
1009119-65-6