D-Phenylalanine
Synonym(s):(R)-2-Amino-3-phenylpropionic acid
- CAS NO.:673-06-3
- Empirical Formula: C9H11NO2
- Molecular Weight: 165.19
- MDL number: MFCD00004270
- EINECS: 211-603-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
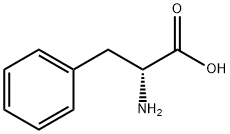
What is D-Phenylalanine?
Description
D-Phenylalanine, also known as D-alpha-Amino-beta-phenylpropionic acid, is a kind of non-proteinogenic amino acid. The biological function of D-phenylalanine remains unclear. However, it has certain anti-depressant, analgesic activities and pharmacological activity at niacin receptor II. The mechanism action of its analgesic activity seems to originate from its inhibition of enkephalin degradation by the carboxypeptidase A. Enkephalins are part of your body’s natural pain relief system. When they are broken down by enkephalinase, this contributes to the sensation of pain. D-Phenylalanine is specifically thought to be beneficial for reducing feelings of chronic pain. D-phenylalanine has been used with mixed results to treat chronic pain, including pain caused by rheumatoid arthritis. The primary use of D-phenylalanine as a health supplement is the relief of discomfort.
Chemical properties
White crystalline powder
The Uses of D-Phenylalanine
D-Phenylalanine, the stereoisomer of L-Phenylalanine (P319415) has been used in the synthesis of Schaeffer’s acid analogues as important structures in tuberculostatic design. They exhibit the ability to inhibit Mycobacterium tuberculosis type II dehydroquinase.
Definition
ChEBI: D-phenylalanine is the D-enantiomer of phenylalanine. It is a phenylalanine and a D-alpha-amino acid. It is a conjugate base of a D-phenylalaninium. It is a conjugate acid of a D-phenylalaninate. It is an enantiomer of a L-phenylalanine. It is a tautomer of a D-phenylalanine zwitterion.
General Description
D-phenylalanine appears as needles or prisms.
Air & Water Reactions
Water soluble. Aqueous solutions are weakly acidic.
Reactivity Profile
D-alpha-Amino-beta-phenylpropionic acid may be light sensitive. D-alpha-Amino-beta-phenylpropionic acid reacts with strong oxidizing agents, acids and bases. . Act as weak acids in solution.
Fire Hazard
Flash point data for D-alpha-Amino-beta-phenylpropionic acid are not available, however D-alpha-Amino-beta-phenylpropionic acid is probably combustible.
Pharmacokinetics
D-Phenylalanine is the synthetic dextro isomer of phenylalanine, an essential amino acid with anti-depressant and analgesic activities. D-Phenylalanine is converted into tyrosine and tyrosine in turn is converted into L-dopa, norepinephrine, and epinephrine, three key neurotransmitters. As a result this agent is associated with elevated levels of the neurotransmitters dopamine and norepinephrine in the brain, which may alleviate symptoms of depression. In addition, as an inhibitor of enkephalinase, which metabolizes endorphins, D-phenylalanine may be used to treat chronic pain through blocking the break down of endorphins (natural pain killers).
Safety Profile
Mildly toxic by intraperitoneal route. Human systemic effects by ingestion: nausea, hypermotility, diarrhea. When heated to decomposition it emits toxic fumes of NOx.
References
https://www.alfa.com/en/catalog/A10572/
https://en.wikipedia.org/wiki/Phenylalanine
Christianson, D. W., et al. "Binding of D-phenylalanine and D-tyrosine to carboxypeptidase A. " Journal of Biological Chemistry264.22(1989):12849-53.
Properties of D-Phenylalanine
| Melting point: | 273-276 °C(lit.) |
| Boiling point: | 293.03°C (rough estimate) |
| alpha | 33.5 º (c=2, H2O) |
| Density | 1.1603 (rough estimate) |
| refractive index | 34 ° (C=2, H2O) |
| storage temp. | Store at RT. |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Powder |
| pka | 2.2(at 25℃) |
| color | White to off-white |
| Water Solubility | 27 g/L (20 ºC) |
| Merck | 14,7271 |
| BRN | 2804068 |
| Stability: | Stable. Incompatible with strong oxidizing agents, acids, bases. |
| CAS DataBase Reference | 673-06-3(CAS DataBase Reference) |
| EPA Substance Registry System | D-Phenylalanine (673-06-3) |
Safety information for D-Phenylalanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-Phenylalanine
| InChIKey | COLNVLDHVKWLRT-MRVPVSSYSA-N |
D-Phenylalanine manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 (R)-Phenylalanine 99%View Details
(R)-Phenylalanine 99%View Details -
 D-Phenylalanine, 98% 99%View Details
D-Phenylalanine, 98% 99%View Details
673-06-3 -
 D-Phenylalanine extrapure CHR CAS 673-06-3View Details
D-Phenylalanine extrapure CHR CAS 673-06-3View Details
673-06-3 -
 D-Phenylalanine CAS 673-06-3View Details
D-Phenylalanine CAS 673-06-3View Details
673-06-3 -
 D-Phenyl alanine CAS 673-06-3View Details
D-Phenyl alanine CAS 673-06-3View Details
673-06-3 -
 D-Phenylalanine CAS 673-06-3View Details
D-Phenylalanine CAS 673-06-3View Details
673-06-3 -
 D-Phenylalanine 98% CAS 673-06-3View Details
D-Phenylalanine 98% CAS 673-06-3View Details
673-06-3 -
 D-Phenylalanine CAS 673-06-3View Details
D-Phenylalanine CAS 673-06-3View Details
673-06-3
