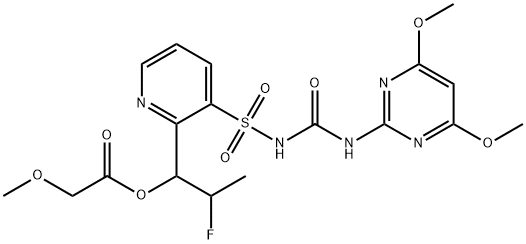
Cyclosulfamuron
Synonym(s):N-{{[2-(Cyclopropylcarbonyl)phenyl]amino}sulfonyl}-N′-(4,6-dimethoxy-2-pyrimidinyl)urea
- CAS NO.:136849-15-5
- Empirical Formula: C17H19N5O6S
- Molecular Weight: 421.43
- MDL number: MFCD03791028
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:30
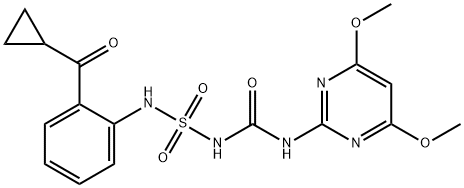
What is Cyclosulfamuron?
The Uses of Cyclosulfamuron
Cyclosulfamuron can be used in analytical study of importance of optimizing chromatographic conditions and mass spectrometric parameters for supercritical fluid chromatography?/mass spectrometry.
Definition
ChEBI: Cyclosulfamuron is a sulfonamide.
Agricultural Uses
Cyclosulfamuron (AC 322140) herbicide was launched in 1997 by American Cyanamid and is marketed by BASF for control of a wide range of broadleaf weeds and sedge species in rice, wheat, and barley. Rice weeds controlled with greater 90% efficiency at an application rate of 45–60 g a.i. ha−1 include Cyperus serotinus, C. difformis, Elatine triandra, Eleocharis congesta, E. kuroguwai, Lindernia annua, L. procumbens, M. vaginalis, Rotala indica, Sagittaria pygmaea, S. trifolia, and Scirpus juncoides. Selectivity in the rice paddy is achieved due to various factors, including rapid metabolic degradation of the herbicide in rice shoots, placement of rice seedlings during transplanting, and the compound’s soil‐binding properties, which retain cyclosulfamuron in the upper soil layer of the paddy.
In rice, cyclosulfamuron is commercialized under the trade name “Ichiyonmaru” and “Saviour.” In combinations with daimuron and cafenstrole, it is sold as “Nebiros” and in combination with pentoxazone as “Utopia.” “Shakariki” is the trade name for the mixture with esprocarb. “Kanetsugu‐Radical” is a combination of cyclosulfamuron and pretilachlor that was recently introduced in Japan in 2015.
At application rates of 25–50 g a.i. ha−1, cyclosulfamuron can also be used in cereal crops for pre‐ and post‐emergent control of several important broadleaf weeds, such as Veronica persica, V. hederifolia, G. aparine, Matricaria spp., and P. convolvulus.
Cyclosulfamuron cannot be synthesized by any of the general methods depicted in Scheme 2.2.1. From the methods reported in the patent literature, Brady et al. describe a straightforward reaction of 2‐amino‐4,6‐dimethoxypyrimidine with chlorosulfonylisocyanate (CSI) at 0 °C with a mixture of 2‐aminophenyl cyclopropyl ketone and triethylamine to yield 70% of the desired herbicide. The synthesis of cyclosulfamuron and its intermediate products was also reported in 2005 by Tan et al.
Properties of Cyclosulfamuron
| Melting point: | 162.0℃ |
| Density | 1.532±0.06 g/cm3(Predicted) |
| storage temp. | 0-6°C |
| solubility | Solubility in organic solvents (g/l at 20 °C) Acetone 21.0 Ethyl acetate 5.0 Dichloromethane 50.0 n‐Hexane <0.001 Methanol 1.5 Toluene 1 |
| pka | Dissociation constant (pKa at 20 °C) 5.04 |
| form | neat |
| Water Solubility | Solubility in water (g/l at 25 °C): 0.001 (pH 5) 0.003 (pH 6) 0.006 (pH 7) 0.032 (pH 8). |
| BRN | 9011949 |
Safety information for Cyclosulfamuron
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cyclosulfamuron
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
