CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
- CAS NO.:35166-78-0
- Empirical Formula: C7H13BrMg
- Molecular Weight: 201.39
- MDL number: MFCD02260210
- EINECS: 233-305-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
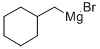
What is CYCLOHEXYLMETHYLMAGNESIUM BROMIDE?
Chemical properties
Colorless to yellow to brown liquid
The Uses of CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
Reactant involved in:• ;Isomerization polymerization of alkenylcyclohexanes1• ;Intramolecular rearrangements of vinylidenecyclopropanes2• ;Cycloisomerization of cyclic and acyclic enynes3Additionally reactant involved in synthesis of biologically active molecules including:• ;Dipeptidyl boronic acid proteasome inhibitors4• ;Substituted 1,3-dihydroindole-2-ones with antitumor effects5• ;Sulfur-free transition state nucleoside analog inhibitors of methylthioadenosine nucleosidase and phosphorylase6
The Uses of CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
Reactant involved in:
- Isomerization polymerization of alkenylcyclohexanes
- Intramolecular rearrangements of vinylidenecyclopropanes
- Cycloisomerization of cyclic and acyclic enynes
Additionally reactant involved in synthesis of biologically active molecules including:
- Dipeptidyl boronic acid proteasome inhibitors
- Substituted 1,3-dihydroindole-2-ones with antitumor effects
- Sulfur-free transition state nucleoside analog inhibitors of methylthioadenosine nucleosidase and phosphorylase
Properties of CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
| Density | 0.966 g/mL at 25 °C |
| Flash point: | -17 °C |
| form | Solution |
| color | Colorless to yellow to brown |
Safety information for CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for CYCLOHEXYLMETHYLMAGNESIUM BROMIDE
CYCLOHEXYLMETHYLMAGNESIUM BROMIDE manufacturer
Sainor Laboratories Pvt Ltd Unit III
1Y
Phone:+91-9182033729
Whatsapp: +91-9666644167
product: 35166-78-0 (Cyclohexylmethyl)magnesium bromide 0.5M in THF 0.5M
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 35166-78-0View Details
35166-78-0View Details
35166-78-0 -
 (Cyclohexylmethyl)magnesium bromide solution CAS 35166-78-0View Details
(Cyclohexylmethyl)magnesium bromide solution CAS 35166-78-0View Details
35166-78-0 -
 35166-78-0 (Cyclohexylmethyl)magnesium bromide 0.5M in THF 0.5MView Details
35166-78-0 (Cyclohexylmethyl)magnesium bromide 0.5M in THF 0.5MView Details
35166-78-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
