Crisaborole
Synonym(s):4-[(1,3-Dihydro-1-hydroxy-2,1-benzoxaborol-5-yl)oxy]benzonitrile;5-(4-Cyanophenoxy)-1,3-dihydro-1-hydroxy-[2,1]-benzoxaborole;5-(4-Cyanophenoxy)-2,3-dihydro-1-hydroxy-2,1-benzoxaborole;AN2728
- CAS NO.:906673-24-3
- Empirical Formula: C14H10BNO3
- Molecular Weight: 251.05
- MDL number: MFCD17169940
- EINECS: 250-635-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
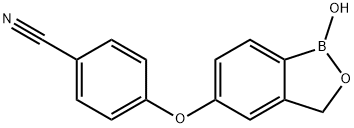
What is Crisaborole?
Absorption
Crisaborole achieves systemic concentrations within 8 days of twice-daily topical administration. Due to its low systemic absorption, it carries a reduced risk for inducing systemic side effects.
Toxicity
Hypersensitivity reactions, such as contact urticaria, can occur with the use of this treatment, and it is advised to discontinue the treatment if such reactions are observed. There is no evidence to suggest that it has mutagenic or clastogenic potential, nor does it appear to have any altered effects on fertility. In terms of oral toxicity, the LD50 value for rats is greater than 500mg/kg, indicating a relatively low toxicity level.
Description
Crisaborole is a phosphodiesterase- 4 (PDE4) inhibitor that was approved by the USFDA for the treatment of mild to moderate atopic dermatitis (AD) in patients aged two years and older. The drug, which was developed by Anacor Pharmaceuticals and later acquired and marketed by Pfizer, is delivered as a 2% ointment, which is applied topically. The unique employment of boron within the chemical structure of the drug is designed to enable selective engagement of PDE4 (an enzyme involved in the conversion of cAMP into AMP, which signals for downstream inflammatory events), effective penetration of the drug through human skin, and rapid clearance to limit systemic circulation.
The Uses of Crisaborole
A series of phenoxy benzoxaboroles were synthesized and screened for their inhibitory activity against PDE4 and cytokine release. 5-?(4-?Cyanophenoxy)?-?2,?3-?dihydro-?1-?hydroxy-?2,?1-?benzoxaborole (AN2728) showed potent activity both in vitro and in vivo. AN2728 is being studied as an anti-inflammatory agent.
Background
Crisaborole, an innovative oxaborole drug, was approved by the FDA on December 14, 2016, and is marketed under the brand name Eucrisa. It is a topical treatment for mild to moderate atopic dermatitis, offering a non-steroidal alternative. This treatment is effective for patients aged 2 years and older, improving disease severity, reducing the risk of infection, and alleviating the signs and symptoms associated with atopic dermatitis. With a good safety profile, crisaborole reduces local skin inflammation and helps prevent the exacerbation of the disease.
Indications
Intended for the topical treatment of mild to moderate atopic dermatitis in patients 2 years of age and older.
Definition
ChEBI: Crisaborole is a member of the class of benzoxaboroles that is 5-hydroxy-1,3-dihydro-2,1-benzoxaborole in which the phenolic hydrogen has been replaced by a 4-cyanophenyl group. A phosphodiesterase 4 inhibitor that is used for treatment of mild to moderate atopic dermatitis in children and adults. It has a role as a phosphodiesterase IV inhibitor, an antipsoriatic and a non-steroidal anti-inflammatory drug. It is a benzoxaborole, an aromatic ether and a nitrile. ?
Biochem/physiol Actions
Crisaborole is a non-steroidal anti-inflammatory phosphodiesterase 4 (PDE4) inhibitor. Crisaborole has an IC50 value of 490 nM for PDE4 with similar IC50 values for release of cytokines TNF-α, IL-2, and IFN-γ, and shows little inhibition against other PDE isozymes. Crisaborole has been approved as a topical treatment for atopic dermatitis.
Pharmacokinetics
Crisaborole has broad-spectrum anti-inflammatory activity by mainly targeting phosphodiesterase 4 (PDE4) enzyme that is a key regulator of inflammatory cytokine production. As this enzyme is expressed in keratinocytes and immune cells, crisaborole mediates an anti-inflammatory effect on almost all inflammatory cells. Topical application of this drug is useful as it potentiates the localization of this drug in the skin and this anti-inflammatory activity is in the low micromolar range.
Side Effects
EUCRISA may cause side effects.
Allergic reactions. EUCRISA may cause allergic reactions at or near the application site. These can be serious and may include hives, itching, swelling, and redness.
www.eucrisa.com
Synthesis
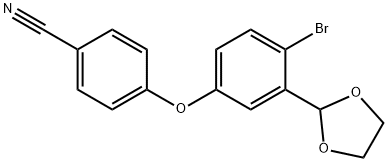
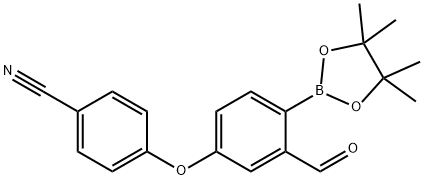
Several disclosures describing synthetic approaches to ethereal boron-containing anti-inflammatory compounds have been published by Anacor. Commercially available bromobenzaldehyde 134 was protected as the corresponding acetal upon subjection to warm ethylene glycol in the presence of catalytic p-toluenesulfonic acid. This was followed by nucleophilic aromatic substitution involving 4-fluorobenzonitrile and subsequent acetal deprotection to furnish diaryl ether 136. Although multiple approaches to crisaborole have been reported from this intermediate diaryl ether 136, the published literature approach involves the following sequence: reduction of the aldehyde with sodium borohydride followed by THP protection to furnish bromobenzene 137, which then underwent lithium-halogen exchange prior to quenching with triisopropyl borate and acidification to arrive at crisaborole (XIII). Alternatively, patents from Anacor describe a general approach employing a Miyaura coupling of 136, enabling the installation of the corresponding pincacol borane, which could then be exposed to reduction conditions using sodium borohydride followed by boric acid wash, aqueous workup, and lyophilization to furnish XIII.
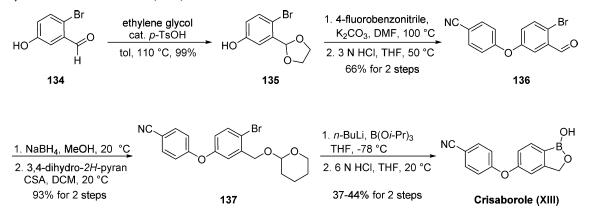
Metabolism
Crisaborole undergoes substantial metabolism to form inactive metabolites. The primary metabolite, known as metabolite 1, is 5-(4-cyanophenoxy)-2-hydroxyl benzylalcohol, which is produced through the process of hydrolysis. This compound can be further metabolized into additional downstream metabolites. Among these, metabolite 2, identified as 5-(4-cyanophenoxy)-2-hydroxyl benzoic acid, is another significant metabolite that is generated through oxidation.
Mode of action
Crisaborole is a Phosphodiesterase 4 Inhibitor. The mechanism of action of crisaborole is as a Phosphodiesterase 4 Inhibitor.
Properties of Crisaborole
| Melting point: | >130°C (softened) |
| Boiling point: | 425.9±55.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 7.00±0.20(Predicted) |
| form | powder |
| color | white to beige |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C14H10BNO3/c16-8-10-1-3-12(4-2-10)19-13-5-6-14-11(7-13)9-18-15(14)17/h1-7,17H,9H2 |
Safety information for Crisaborole
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Crisaborole
| InChIKey | USZAGAREISWJDP-UHFFFAOYSA-N |
| SMILES | C(#N)C1=CC=C(OC2=CC=C3B(O)OCC3=C2)C=C1 |
Crisaborole manufacturer
LUPA PHARMACEUTICALS
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 906673-24-3 Crisaborole 97%View Details
906673-24-3 Crisaborole 97%View Details
906673-24-3 -
 906673-24-3 98%View Details
906673-24-3 98%View Details
906673-24-3 -
 Crisaborole 98%View Details
Crisaborole 98%View Details -
 Crisaborole 906673-24-3 98%View Details
Crisaborole 906673-24-3 98%View Details
906673-24-3 -
 Crisaborole 98%View Details
Crisaborole 98%View Details -
 906673-24-3 Crisaborole 98+View Details
906673-24-3 Crisaborole 98+View Details
906673-24-3 -
 Crisaborole CAS 906673-24-3View Details
Crisaborole CAS 906673-24-3View Details
906673-24-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
