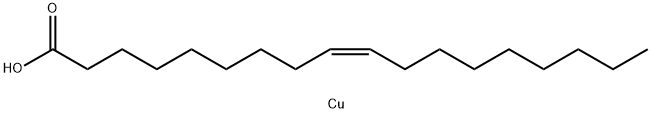
COPPER(II) OLEATE
- CAS NO.:1120-44-1
- Empirical Formula: C18H34CuO2
- Molecular Weight: 346.01
- MDL number: MFCD00064999
- EINECS: 214-307-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
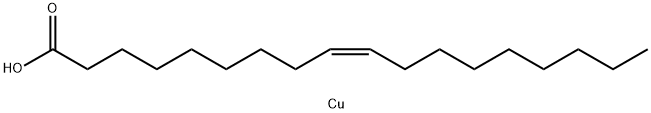
What is COPPER(II) OLEATE?
Chemical properties
Copper(II) oleate is a blue to green solid; can be prepared by reacting the acid with CuO or basic carbonate of Cu; coalesces mercury drops; improves oil combustion by reducing smoke and fumes of burning oil; used as textile fungicide, used in antifouling marine paints [MER06].
The Uses of COPPER(II) OLEATE
In antifouling compositions; as emulsifier and dispersing agent; as antioxidant in lubricating oils; as combustion-improver in fuel oils; as stabilizer for amide polymers; as catalyst.
Preparation
Copper(II) oleate is produced by the precipitation reaction of copper(II) sulfate solutions with sodium oleate. It is insoluble in water. The product of commerce contains between 6% and 9% copper. Copper(II) oleate is used as an emulsifier and dispersant for the control of mildew and as a combustion improver in fuel oils.
Flammability and Explosibility
Not classified
Synthesis
The electrolysis system consisted of a Cu foil (3x1 cm, 0.1 cm thickness) as the anode and graphite rod (6.5 mm diameter, Johnson Matthey Chemicals Ltd.) as the cathode in the presence of ammonium acetate (CH3COONH4) as the supporting electrolyte solution. Prior to synthesis, both anode and cathode were rinsed with distilled water and small amount of acetone to remove any trace organic materials on their surface. A DC power supply (TTi PSU Bench CPX400A) was used throughout the electrochemical synthesis. The acid solution (in ethanol) was mixed with CH3COONH4 solution with the ratio of 1:1 into the electrochemical cell. The electrochemical cell used was a simple and undivided cell with the capacity of 100mL. The reaction was performed at room temperature (~27°C) for 4 hours. Strong stirring (900 rpm) was kept throughout the synthesis process. The resulting complex in blue precipitate was then filtered and washed with distilled water and ethanol to remove the impurities. The precipitate was then dried in a desiccator for 24 hours. Copper(II) oleate can be obtained.
Purification Methods
Crystallise it from diethyl ether. [Beilstein 2 H 465, 2 I 196, 2 I202, 2 II 436, 2 III 1404, 2 IV 1646.]
Toxicity evaluation
Toxicity of Copper(II) oleate was demonstrate in experiments on S.mansoni cercariae. 1 mL of Copper(II) oleate (20% in ether) was put in a tet tube containing 10 mL of aerated tap water. Test tubes were allowed to stand for 30 minutes with occasional shaking. The water was then poured off and filtered. 1 mL of filtrate from each tube was placed in a separate Syracuse watch glass. Water saturated with copper oleate killed all the cercariae in 30 minutes.
Properties of COPPER(II) OLEATE
| Boiling point: | 211.9℃[at 101 325 Pa] |
| Density | 971[at 20℃] |
| vapor pressure | 0.616Pa at 20℃ |
| solubility | insoluble in H2O; slightly soluble in ethanol; soluble in ethyl ether |
| form | blue-green solid |
| color | blue-green |
| Water Solubility | insoluble H2O; slightly soluble alcohol; soluble ether [MER06] |
| Dielectric constant | 2.8(20℃) |
| EPA Substance Registry System | Cupric oleate (1120-44-1) |
Safety information for COPPER(II) OLEATE
Computed Descriptors for COPPER(II) OLEATE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1