Copper Peptide
- CAS NO.:49557-75-7
- Empirical Formula: C14H24N6O4
- Molecular Weight: 340.38
- MDL number: MFCD00036754
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
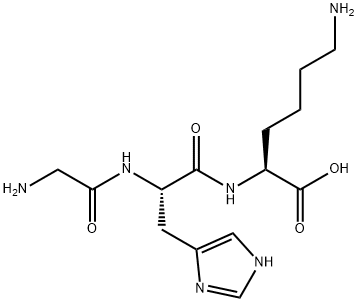
What is Copper Peptide?
Description
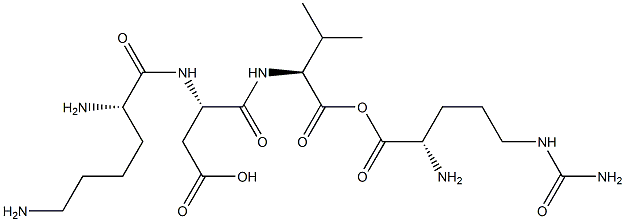
Copper peptide GHK-Cu is a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine. The tripeptide has strong affinity for copper(II) and was first isolated from human plasma. It can be found also in saliva and urine.
The Uses of Copper Peptide
Copper Peptide(GHK-Cu) can be used as skin antiaging agents.
The Uses of Copper Peptide
anti-wrikle
The Uses of Copper Peptide
Glycyl-L-histidyl-L-lysine is a liver cell growth factor and an asynthetic hepatotrophic agent that stimulates hepatic erythropoietic factor production.
Definition
ChEBI: A tripeptide composed of glycine, L-histidine and L-lysine residues joined in sequence.
Pharmaceutical Applications
Glycyl-l-histidyl-l-lysine (GHK) is a tripeptide known for its high binding affinity to Cu2+ and its complex
role in wound healing. The GHK–Cu(II) complex was isolated from human plasma in the 1970s and it was
shown to be an activator for wound healing. GHK–Cu(II) has two main functions: as an anti-inflammatory
agent to protect the tissue from oxidative damage after the injury, and as an activator for wound healing itself
as it activates the tissue remodelling.
The structure of GHK is very similar to that of common drugs used to treat ulcers.
After the initial stages of wound healing are activated, such as blood coagulation and neutrophil invasion, a
second stage of wound healing begins, which includes the population of GHK at the wound, which has a high
affinity to Cu2+. Mast cells, which are located in the skin, secrete GHK, which accumulates Cu2+ and forms
the copper complex GHK–Cu(II) and therefore increases the metal–tripeptide concentration at the wound.
First, GHK–Cu(II) has an anti-inflammatory effect by protecting the tissue from oxidative damage and by
suppressing local inflammatory signals (i.e. cytokine interleukin-1 (IL-1)). Second, GHK–Cu(II) is released
into the blood stream and encourages the production of wound macrophages that support the wound repair
by removing the damaged tissue and secreting a family of several growth factor proteins. GHK–Cu(II) also
hinders fibroblast production of TGF-β-1 and therefore suppresses the scar development. The GHK–Cu(II)
complex also stimulates the growth of blood vessels, neurons and elastin, and, in general, supports most
processes of wound healing.
Side Effects
While Copper Peptide Serum is generally safe to use, you should be aware of potential side effects that may occur. Although rare, some individuals may experience skin irritation or redness after applying the serum. This can be due to sensitivity to the ingredients or an allergic reaction. Another potential side effect of Copper Peptide Serum is increased sensitivity to sunlight. The serum can make your skin more prone to sunburn.
Properties of Copper Peptide
| Melting point: | >144°C (dec.) |
| Boiling point: | 831.0±65.0 °C(Predicted) |
| Density | 1.324 |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 3.11±0.10(Predicted) |
| color | Off-White |
| Stability: | Hygroscopic |
Safety information for Copper Peptide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Copper Peptide
| InChIKey | MVORZMQFXBLMHM-QWRGUYRKSA-N |
| SMILES | C(O)(=O)[C@H](CCCCN)NC(=O)[C@H](CC1N=CNC=1)NC(=O)CN |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Glycyl-l-histidyl-l-lysine 95% CAS 49557-75-7View Details
Glycyl-l-histidyl-l-lysine 95% CAS 49557-75-7View Details
49557-75-7 -
 Glycyl-L-histidyl-L-lysine CASView Details
Glycyl-L-histidyl-L-lysine CASView Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4