COPPER FORMATE
- CAS NO.:544-19-4
- Empirical Formula: C2H2CuO4
- Molecular Weight: 153.58
- MDL number: MFCD00050829
- EINECS: 208-865-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
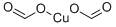
What is COPPER FORMATE?
Chemical properties
three forms of anhydrous formate exist: powder(s) blue, turquoise or royal blue crystal(s) [MER06].
Copper(II) formate is a royal blue material that obtains by crystallization from 75-85°C solutions. Crystallization from solutions at temperatures of 50-60°C results in the formation of a metastable dihydrate. A tetrahydrate can be produced by crystallization at lower temperatures.

The Uses of COPPER FORMATE
Copper formate is used for the control of bacteria and mildew in cellulosic materials.
Preparation
Copper(II) formate is produced by dissolution of copper(II) oxide in hot formic acid or by the reaction of copper(II) carbonate or hydroxide with formic acid. It can also be produced by aeration of hot formic acid over copper metal.
General Description
Blue crystalline powder. Sinks and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as COPPER FORMATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
INHALATION: May cause nasal congestion. EYES: May cause conjunctivitis. SKIN: Irritation. INGESTION: Irritation.
Properties of COPPER FORMATE
| Melting point: | 130°C |
| Density | 1,831 g/cm3 |
| solubility | insoluble in organic solvents |
| form | Powder |
| color | green to blue |
| Specific Gravity | 1.831 |
| Water Solubility | 12.5g/100mL H2O [CRC10]; insoluble most organic solvents [MER06] |
| Merck | 14,2639 |
| EPA Substance Registry System | Formic acid, copper(2+) salt (2:1) (544-19-4) |
Safety information for COPPER FORMATE
Computed Descriptors for COPPER FORMATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![bis[4-hydroxysalicylato(2-)-O1,O2]cuprate(2-)](https://img.chemicalbook.in/CAS/GIF/32628-05-0.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1