Colestipol HCl
- CAS NO.:26658-42-4
- Empirical Formula: C11H28ClN5O
- Molecular Weight: 281.83
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-25 18:01:07
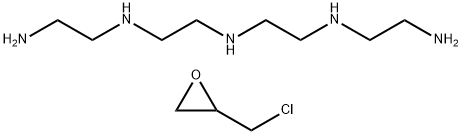
What is Colestipol HCl?
Absorption
Colestipol is hydrophilic, but it is virtually water-insoluble (99.75%) . This water insolubility, combined with the high molecular weight polymer in colestipol basically means the agent and the complexes it forms when it binds with bile acids are not absorbed . The action of colestipol is ultimately limited to the lumen of the gastrointestinal tract . It binds bile acids in the intestinal lumen and causes them to be excreted in the feces together with the polymer . When the enterohepatic circulation of bile acids is interrupted, cholesterol conversion to bile acids is enhanced and plasma cholesterol levels are thereby lowered .
Toxicity
Overdosage with colestipol has not been reported . Regardless, in the case of overdosage, the chief potential harm would be obstruction of the gastrointestinal tract . The location of such potential obstruction, the degree of obstruction and the presence or absence of normal gut motility would determine treatment .
No clinical data are available on the use of colestipol in pregnant women and during lactation . Colestipol does not appear to be absorbed systematically . Due to its known interference with absorption of fat-soluble vitamins, the use of colestipol in pregnancy or lactation or by women of childbearing potential requires that the benefits of drug therapy be weighed against the possible hazards to the mother and the child .
There are no data on the effect of colestipol on fertility in humans .
The use of colestipol in children is limited . Clinical trials conducted in children with colestipol ranules have usually employed doses of 5 to 20 g/day . The National Cholesterol Education Program (NCEP) Expert Panel recommends drug therapy be considered in children 10 years or older, who have previously undergone an adequate trial of diet therapy but still have unacceptably high serum cholesterol levels . In certain situations where a young child has extremely high serum cholesterol levels, drug treatment may even be initiated before 10 years of age . If the child is started on drug therapy, a carefully assessed diet therapy should also be continued in order to obtain optimal results . However, the safety of using colestipol tablets in patients under the age of 18 years has not been established . Furthermore, because bile acid sequestrants like colestipol may interfere with the absorption of fat-soluble vitamins, appropriate monitoring of growth and development is essential if colestipol is used in children .
Appropriate studies on the relationship of age to the effects of colestipol have not been performed in the geriatric population . However, patients over 60 years of age may be more likely to experience gastrointestinal side effects, as well as adverse nutritional effects .
Additionally, it has been determined that the oral LD50 in rats is > 1000 mg/kg .
Originator
Colestid,Upjohn,US,1977
The Uses of Colestipol HCl
Antihyperlipidemic.
Indications
Colestipol is indicated as adjunctive therapy to diet for the reduction of elevated serum total and low-density lipoprotein cholesterol (LDL-C) in patients with primary hypercholesterolemia (a condition that features elevated LDL-C) who do not respond adequately to dietary changes .
Therapy with lipid-altering agents like colestipol should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia . Treatment should begin and continue with dietary therapy . In general, a minimum of six months of intensive dietary therapy and counseling should be carried out prior to initiation of drug therapy such as that with colestipol . Shorter periods may be considered in patients with severe elevations of LDL-C or with definite coronary heart disease .
Although colestipol is effective in all types of hypercholesterolemia, some regional prescribing information note in particular that it is medically most appropriate in patients with Fredrickson's type II hyperlipoproteinemia . Nevertheless, in patients with combined hypercholesterolemia and hypertriglyceridemia, although colestipol may be helpful in reducing elevated cholesterol, it is not formally indicated where hypertriglyceridemia is the abnormality of greatest concern .
Background
Bile acid sequestrants like colestipol have been in use since the 1970s. And even though such an agent may very well be useful in reducing elevated cholesterol levels and decreasing the risk for atherosclerotic vascular disease due to hypercholesterolemia, colestipol is still generally only employed as an adjunct therapy and the relatively physical nature of its pharmacological activity sometimes limits its usefulness.
In particular, as colestipol's general mechanism of action ultimately results in the decreased absorption and enhanced secretion of bile acids and lipids in the feces, patients who take complicated medication regimens, experience constipation or biliary obstruction, etc. may not be good candidates for using the agent owing to its physical effects on the gut.
Alternatively, colestipol predominantly elicits its activities within the gut environment because it undergoes little absorption and metabolism. The resultant lack of systemic exposure consequently means the medication generally demonstrates very few adverse effects inside the body.
Manufacturing Process
Into a 1,000 gallon, jacketed, glass-lined reactor equipped with baffles and a two-speed (67 and 135 rpm) reversed impeller is introduced 200 g of Richonate 60B (a 60% aqueous slurry of sodium salts of alkylbenzenesulfonic acids) and 364 liters of deionized water, followed by 90.5 kg of tetraethylenepentamine rinsed in with 5 gallons of toluene. The solution is stirred at the low speed and then 500 gallons of toluene are added to form a dispersion. To the stirred dispersion is added 109 kg of epichlorohydrin, rinsed in with 5 gallons of toluene, and the resulting mixture is heated at reflux for two hours. The reaction mixture is cooled to about 20°C and then treated with 58.5 kg of a filtered 50% aqueous solution of sodium hydroxide. The mixture is removed from the reactor and filtered, and the copolymer is collected and dried by treating it first with hot (75°C to 80°C) filtered nitrogen and then with an 80°C air stream. The resulting crude product is returned to the reactor, washed extensively with filtered deionized water (at the low speed), dried with an 80°C air stream and blended until homogeneous to give about 155 kg of a dry tetraethylenepentamine-epichlorohydrin copolymer hydrochloride, particle diameter 0.002-0.02 inch.
brand name
Colestid (Pharmacia & Upjohn).
Therapeutic Function
Antihyperlipoproteinemic
Pharmacokinetics
Cholesterol is the major, and probably the sole precursor of bile acids . During normal digestion, bile acids are secreted via the bile from the liver and gall bladder into the intestines . Bile acids emulsify the fat and lipid materials present in food, thus facilitating absorption . A major portion of the bile acids secreted is reabsorbed from the intestines and returned via the portal circulation to the liver, thus completing the enterohepatic cycle . Only very small amounts of bile acids are found in normal serum .
Colestipol hydrochloride binds bile acids in the intestine forming a complex that is excreted in the feces . This nonsystemic action results in a partial removal of the bile acids from the enterohepatic circulation, preventing their reabsorption . Since colestipol hydrochloride is an anion exchange resin, the chloride anions of the resin can be replaced by other anions, usually those with a greater affinity for the resin than the chloride ion .
Metabolism
Colestipol is not absorbed into the systemic circulation nor is it hydrolyzed by any digestive enzymes .
Properties of Colestipol HCl
| EPA Substance Registry System | Tetraethylenepentamine epichlorohydrin polymer (26658-42-4) |
Safety information for Colestipol HCl
Computed Descriptors for Colestipol HCl
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate 4-AMINO-2-CHLOROPYRIDINE Benzyl {1-[4-(4- Fluorobenzylcarbamoyl)-5-hydroxy-1- methyl-6-oxo-1,6-dihydropyrimidin-2- yl]-1-methylethyl}-carbamate R,R-Chiraphite 4-BROMO-2-METHYLBENZOIC ACID N-Boc-1,4-butanediamine 2-AMINO-6-METHOXYBENZOTHIAZOLE Cyaclopentane carboxylic acid 3-Nitropyrazole 4-Pyrazolecarboxylic acid 1-Nitropyrazole 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine 2-Bromo-6-methyl-4-nitrobenzenamine 2-Chloro-5-fluoro-3-methylphenol (R)-3-Amino-4-(2,4-difluorophenyl)butanoic acid hydrochlorideYou may like
-
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 3112-85-4 98%View Details
3112-85-4 98%View Details
3112-85-4 -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 2058-74-4 1-methylindoline-2,3-dione 98%View Details
2058-74-4 1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8 -
 3-amino-3-phenylpropanoic acid 98%View Details
3-amino-3-phenylpropanoic acid 98%View Details
614-19-7 -
 (2,2-diethoxyethyl)methylamine 20677-73-0 98%View Details
(2,2-diethoxyethyl)methylamine 20677-73-0 98%View Details
20677-73-0