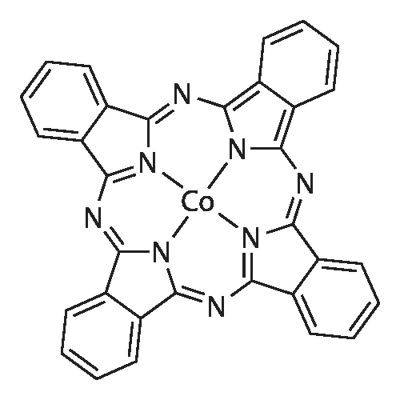
Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
Synonym(s):(Hexadecafluorophthalocyaninato)cobalt;Cobalt hexadecafluorophthalocyanine
- CAS NO.:52629-20-6
- Empirical Formula: C32CoF16N8
- Molecular Weight: 859.32
- MDL number: MFCD00274641
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-28 09:21:42
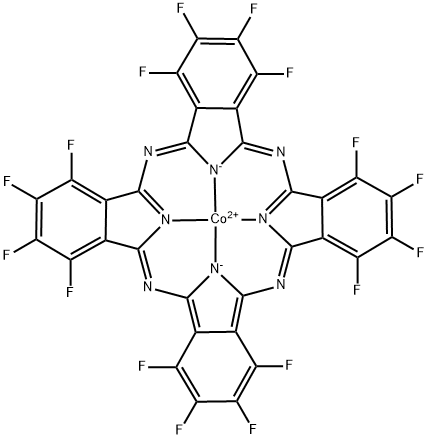
What is Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine?
Chemical properties
Green solid
The Uses of Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
The graphite electrode surface onto which Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine (CoPcF16; CoIIHFPC)is adsorbed displays a strong electrocatalytic activity toward O2 reduction. CoIIHFPC instead of CoPc(NH2)4, as the catalyst for electrochemical reduction of O2 because CoPcF16 contains electro-withdrawing groups, and thus, has a higher catalytic activity and is more stable for O2 reduction compared with CoPc(NH2)4. The OH- concentration has a very strong effect on the reduction process. Higher OH- concentration could stabilize O-2 ion[1-3].
References
[1] C. Song. “Temperature Dependence of Oxygen Reduction Catalyzed by Cobalt Fluoro-Phthalocyanine Adsorbed on a Graphite Electrode.” Fuel Cells 7 1 (2007): 9–15.
[2] Lanqun Mao . “A novel alkaline air electrode based on a combined use of cobalt hexadecafluoro-phthalocyanine and manganese oxide.” Electrochimica Acta 49 15 (2004): Pages 2515-2521.
[3] Lanqun Mao . “A novel alkaline air electrode based on a combined use of cobalt hexadecafluoro-phthalocyanine and manganese oxide.” Electrochimica Acta 49 15 (2004): Pages 2515-2521.
Properties of Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
| Melting point: | >300 °C (lit.) |
Safety information for Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Cobalt(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
| InChIKey | OFILAZNRYPZFNX-UHFFFAOYSA-N |
| SMILES | FC1C(=C(F)C(F)=C2C3=NC4C5=C(C(F)=C(F)C(F)=C5C5=NC6=C7C(=C(F)C(F)=C(F)C7=C7N=C8C9=C(C(F)=C(F)C(F)=C9C9=N8[Co+2](N=45)([N-]76)[N-]3C(=N9)C=12)F)F)F)F |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
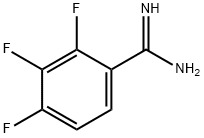
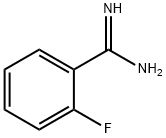
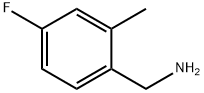
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1