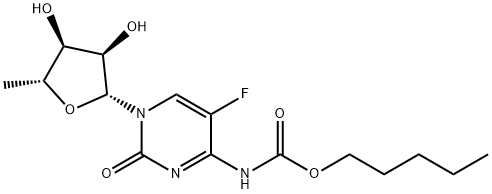
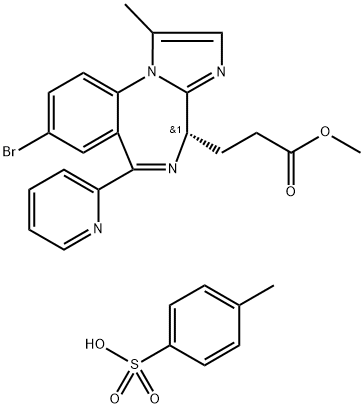
CNS 7056
- CAS NO.:308242-62-8
- Empirical Formula: C21H19BrN4O2
- Molecular Weight: 439.31
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:44:01
What is CNS 7056?
Absorption
The Cmax and AUC0-inf following intravenous administration of 0.01 to 0.5 mg/kg were 189 to 6,960 ng/mL and 12.1 to 452 ng?h/mL, respectively, and appear to be relatively dose proportional. The Tmax of the inactive CNS7054 metabolite is approximately 20-30 minutes and its AUC0-inf ranges from 231 to 7,090 ng?h/mL.
Toxicity
Symptoms of remimazolam overdoses, as with other benzodiazepines, are likely to be consistent with its adverse effect profile and may therefore involve significant CNS depression, respiratory depression, ataxia, and hypotension. The benzodiazepine receptor antagonist flumazenil may be used for reversal of the sedative effects associated with benzodiazepine overdose, though it is not a substitute for proper supportive care.
The Uses of CNS 7056
Vibegron is a potent and selective β3 Adrenergic receptor agonist for the treatment of overactive bladder.
Background
Remimazolam is an ultra short-acting benzodiazepine used in the induction and maintenance of sedation during short (<30 minute) procedures. Recent trends in anesthesia-related drug development have touted the benefits of so-called "soft drugs" - these agents, such as remifentanil, are designed to be metabolically fragile and thus susceptible to rapid biotransformation and elimination as inactive metabolites. These "soft drugs" are useful in the context of surgical procedures, wherein a rapid onset/offset is desirable, enabling anesthesiologists to manipulate drug concentrations as needed. Remimazolam was the first "soft" benzodiazepine analog to be developed and was approved for use by the FDA in July 2020 under the brand name Byfavo.
Indications
Remimazolam is indicated for the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less.
Pharmacokinetics
Remimazolam modulates the effects of GABA(A) receptors in order to enhance the effects of GABA. It is considered an "ultra short-acting" benzodiazepine that achieves peak sedation within 3 to 3.5 minutes following intravenous administration, a property that makes it desirable for use during short procedures. Hepatic impairment can result in elevated serum levels of remimazolam - patients with severe hepatic impairment should be carefully titrated to effect. As of its approval date, remimazolam has not received a scheduling action by the DEA under the Controlled Substances Act. As benzodiazepines as a class have been implicated in the development of drug dependence and have a known potential for abuse, remimazolam should be used with caution in patients with a history of drug dependence or abuse.
Metabolism
Remimazolam does not appear to undergo biotransformation via hepatic cytochrome P450 enzymes, nor does it induce or inhibit these enzymes. Its primary route of metabolism is hydrolysis via hepatic carboxylesterase-1 (CES1) to yield the inactive CNS7054 metabolite, which then undergoes glucuronidation and hydroxylation prior to elimination. CNS7054 possesses a 300-fold lesser affinity for GABA(A) receptors as compared to the parent drug.
Properties of CNS 7056
| Boiling point: | 583.5±60.0 °C(Predicted) |
| Density | 1.48±0.1 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 5.58±0.60(Predicted) |
| form | Solid |
| color | Off-White to Pale Yellow |
Safety information for CNS 7056
Computed Descriptors for CNS 7056
CNS 7056 manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Vibegron 98%View Details
Vibegron 98%View Details -
 Remimazolam CAS 308242-62-8View Details
Remimazolam CAS 308242-62-8View Details
308242-62-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1