CITRONELLOL
- CAS NO.:26489-01-0
- Empirical Formula: C10H20O
- Molecular Weight: 156.27
- MDL number: MFCD00002935
- EINECS: 203-375-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:36
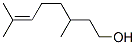
What is CITRONELLOL?
Chemical properties
The enantiomers (3R)-(+)-citronellol and (3S)-(?)-citronellol
occur in many essential oils.
(?)-Citronellol isolated from natural sources is often named rhodinol. At
present, the name rhodinol is also used for the isopropenyl isomer α-citronellol
or a mixture of the two isomers.
In many natural products, citronellol occurs as a mixture of its two enantiomers;
the pure (+) or (?) formis seldom found. (?)-Citronellol is the predominant enantiomer
in geranium and rose oils, both of whichmay contain up to 50% citronellols.
Citronellol is a colorless liquid with a sweet rose-like odor. The odor of (?)-
citronellol is more delicate than that of (+)-citronellol.
Citronellol undergoes the typical reactions of primary alcohols. Compared with
geraniol, which contains one more double bond, citronellol is relatively stable.
Citronellol is converted into citronellal by dehydrogenation or oxidation; hydrogenation
yields 3,7-dimethyloctan-l-ol. Citronellyl esters are easily prepared by
esterification with acid anhydrides.
Preparation
(?)-Citronellol is still obtained mainly from geranium oil by
saponification followed by fractional distillation (“rhodinol”). Although of high
odor quality, this grade does not possess the true (?)-citronellol odor due to
impurities. Much larger quantities of (+)-citronellol and racemic citronellol are
used and are prepared by partial or total synthesis.
1) Synthesis from citronellal: Citronellal can be hydrogenated to citronellol
by the use of special catalysts and/or special hydrogenation techniques,
for example, [122]. The citronellal that is used as the starting material
may originate from synthetic production or from isolation of essential
oils. Citronellal from citronella oil yields (+)-citronellol; the corresponding
material from citronellal from Eucalyptus citriodora oil is racemic. Pure
(+)-citronellol is also obtained from (+)-citronellal, which is produced as an
intermediate of (?)-menthol. By this asymmetric technology,
pure (?)-citronellal and, therefore, pure (?)-citronellol are also available.
2) Synthesis of racemic or slightly dextrorotatory citronellol from geraniol
fractions of essential oils: This citronellol is produced by catalytic hydrogenation of saponified geraniol fractions (also containing (+)-
citronellol) obtained from Java citronella oil, followed by fractional
distillation. Selective hydrogenation of the double bond in the 2-position
of geraniol in geraniol–citronellol mixtures isolated from essential oils
can be achieved by using Raney cobalt as a catalyst; overhydrogenation to
3,7-dimethyloctan-l-ol can be largely avoided by this method.
3) Synthesis of racemic citronellol from synthetic geraniol, nerol, or citral: A considerable
amount of commercial synthetic racemic citronellol is produced by
partial hydrogenation of synthetic geraniol and/or nerol. Another starting
material is citral, which can be hydrogenated, for example, in the presence
of a catalyst system consisting of transition metals and amines.
4) Preparation of (?)-citronellol from optically active pinenes: (+)-cis-Pinane is
readily synthesized by hydrogenation of (+)-α-pinene or (+)-β-pinene and is
then pyrolyzed to give (+)-3,7-dimethyl-1,6-octadiene. This compound can
be converted into (?)-citronellol (97% purity) by reaction with triisobutylaluminumor
diisobutylaluminumhydride, followed by air oxidation and hydrolysis
of the resulting aluminum alcoholate.
Definition
ChEBI: Citronellol is a monoterpenoid that is oct-6-ene substituted by a hydroxy group at position 1 and methyl groups at positions 3 and 7. It has a role as a plant metabolite.
Contact allergens
L-Citronellol is a constituent of rose and geranium oils. d-Citronellol occurs in Ceylon and Java citronella oils. As a fragrance allergen, citronellol has to be mentioned by name in cosmetics within the EU.
Properties of CITRONELLOL
| Melting point: | 77-83 °C(lit.) |
| Boiling point: | 225 °C(lit.) |
| Density | 0.857 g/mL at 25 °C(lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | ~0.02 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 209 °F |
| storage temp. | 2-8°C |
| CAS DataBase Reference | 26489-01-0(CAS DataBase Reference) |
Safety information for CITRONELLOL
Computed Descriptors for CITRONELLOL
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran






You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4