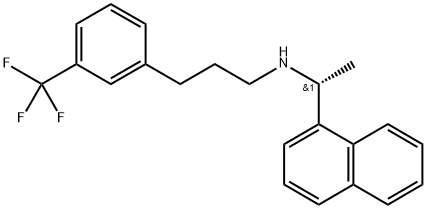
CINACALCET
- CAS NO.:226256-56-0
- Empirical Formula: C22H22F3N
- Molecular Weight: 357.42
- MDL number: MFCD09840270
- EINECS: 682-701-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
What is CINACALCET?
Absorption
Rapidly absorbed following oral administration.
Toxicity
Doses titrated up to 300 mg once daily have been safely administered to patients on dialysis. Overdosage of cinacalcet may lead to hypocalcemia.
Description
Cinacalcet is the first type II calcimimet ic agent approved that improves CaSR sensi Tivity to calcium . When calcium is bound to the CaSR, phospholipase C is act ivated, and the secretion of PTH is inhibited. In the presence of cinacalcet , not only is a drop in PTH levels observed but also a decrease in serum calcium and phosphorous levels.
Chemical properties
Yellow Oil
The Uses of CINACALCET
Cinacalcet is the first calcimimetic drug approved by the United States Food and Drug Administration for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease
The Uses of CINACALCET
macular degeneration therapy
The Uses of CINACALCET
Labeled Cinacalcet, intended for use as an internal standard for the quantification of Cinacalcet by GC- or LC-mass spectrometry.
Indications
For the treatment of secondary hyperparathyroidism in patients with Chronic Kidney Disease who are on hemodialysis or peritoneal dialysis. Also for the treatment of hypercalcemia in patients with parathyroid carcinoma.
Background
Cinacalcet is a calcimimetic sold by Amgen under the trade name Sensipar? in North America and Australia and as Mimpara? in Europe. It is used to treat hyperparathyroidism due to parathyroid tumours or renal failure.
Definition
ChEBI: Cinacalcet is a secondary amino compound that is (1R)-1-(naphthalen-1-yl)ethanamine in which one of the hydrogens attached to the nitrogen is substituted by a 3-[3-(trifluoromethyl)phenyl]propyl group. It has a role as a calcimimetic and a P450 inhibitor. It is a member of naphthalenes, a secondary amino compound and a member of (trifluoromethyl)benzenes.
brand name
Sensipar (Amgen).
Pharmacokinetics
Cinacalcet is a drug that acts as a calcimimetic (i.e. it mimics the action of calcium on tissues). Secondary hyperparathyroidism (HPT) in patients with chronic kidney disease (CKD) is a progressive disease, associated with increases in parathyroid hormone (PTH) levels and derangements in calcium and phosphorus metabolism. Increased PTH stimulates osteoclastic activity resulting in cortical bone resorption and marrow fibrosis. The goals of treatment of secondary hyperparathyroidism are to lower levels of PTH, calcium, and phosphorus in the blood, in order to prevent progressive bone disease and the systemic consequences of disordered mineral metabolism. In CKD patients on dialysis with uncontrolled secondary HPT, reductions in PTH are associated with a favorable impact on bone-specific alkaline phosphatase (BALP), bone turnover and bone fibrosis. Cinacalcet reduces calcium levels by increasing the sensitivity of the calcium sensing receptor to extracellular calcium.
Clinical Use
Cinacalcet hydrochloride is a second-generat ion calcimimetic approved for the treatment of secondary hyperparathyroidism in patients wi th chronic kidney disease on dialysis and for the treatment of hypercalcemia in patients with parathyroid cancer . It can be used alone, with vitamin D, and/or with a phosphate binder .
Side Effects
Common side effects of Cinacalcet are nausea, vomiting, dizziness, diarrhoea and loss of appetite.Cinacalcet can cause hypocalcaemia. Patients taking this medication need to be followed closely for common symptoms associated with hypocalcaemia, including tingling, muscle twitching, cramps, mood changes or irritability. On the ECG, there may be a prolonged QT interval. Patients with a history of congenital long QT syndrome, a family history of long QT syndrome, and other conditions predisposing them to prolonged QT intervals may be at increased risk. PTH oversupport can lead to ankylosing bone disease.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: metabolism inhibited by ketoconazole.
Hormone antagonists: metabolism of tamoxifen to
active metabolite inhibited - avoid.
Tobacco: metabolism increased by tobacco.
Metabolism
Metabolism is hepatic by multiple enzymes, primarily CYP3A4, CYP2D6, and CYP1A2. After administration of a 75 mg radiolabeled dose to healthy volunteers, cinacalcet was rapidly and extensively metabolized via: 1) oxidative N-dealkylation to hydrocinnamic acid and hydroxy-hydrocinnamic acid, which are further metabolized via ?-oxidation and glycine conjugation; the oxidative N-dealkylation process also generates metabolites that contain the naphthalene ring; and 2) oxidation of the naphthalene ring on the parent drug to form dihydrodiols, which are further conjugated with glucuronic acid.
Metabolism
Cinacalcet is rapidly and extensively metabolised by cytochrome P450 isoenzymes CYP3A4 and CYP1A2, by oxidation followed by conjugation. The major circulating metabolites are inactive, and are renally excreted, with 80% of the dose recovered in the urine, and 15% in the faeces.
Properties of CINACALCET
| Boiling point: | 440.9±45.0 °C(Predicted) |
| Density | 1.154±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| pka | 9.19±0.29(Predicted) |
| color | Yellow |
| InChI | InChI=1/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/s3 |
| CAS DataBase Reference | 226256-56-0(CAS DataBase Reference) |
Safety information for CINACALCET
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P330:Rinse mouth. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for CINACALCET
| InChIKey | VDHAWDNDOKGFTD-QEIABERDNA-N |
| SMILES | [C@H](C1=CC=CC2C=CC=CC1=2)(C)NCCCC1C=CC=C(C(F)(F)F)C=1 |&1:0,r| |
CINACALCET manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
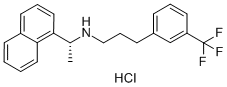
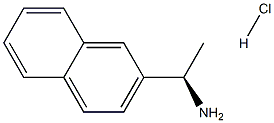

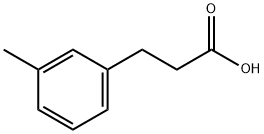
![1-NaphthaleneMethanaMine, 7,8-dihydro-α-Methyl-N-[3-[3-(trifluoroMethyl)phenyl]propyl]-, hydrochloride (1:1), (αR)-](https://img.chemicalbook.in/CAS/20180808/GIF/1020414-33-8.gif)
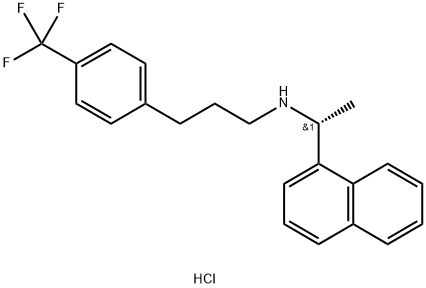
You may like
-
 High Quality Calcium Mimicking Agent Powder CINACALCETView Details
High Quality Calcium Mimicking Agent Powder CINACALCETView Details
226256-56-0 -
 226256-56-0 98%View Details
226256-56-0 98%View Details
226256-56-0 -
 Cinacalcet 99%View Details
Cinacalcet 99%View Details
226256-56-0 -
 Cinacalcet 226256-56-0 98%View Details
Cinacalcet 226256-56-0 98%View Details
226256-56-0 -
 226256-56-0 Cinacalcet 98%View Details
226256-56-0 Cinacalcet 98%View Details
226256-56-0 -
 226256-56-0 98%View Details
226256-56-0 98%View Details
226256-56-0 -
 Cinacalcet 98%View Details
Cinacalcet 98%View Details
226256-56-0 -
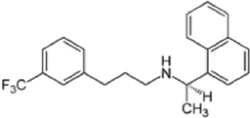 Cinacalcet api cas 226256-56-0View Details
Cinacalcet api cas 226256-56-0View Details
226256-56-0
