Chalcone
- CAS NO.:94-41-7
- Empirical Formula: C15H12O
- Molecular Weight: 208.26
- MDL number: MFCD00003082
- EINECS: 202-330-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
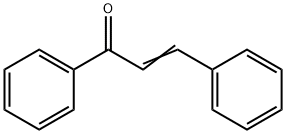
What is Chalcone?
Description
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids.They show antibacterial, antifungal, antitumor and anti-inflammatory properties. They are also intermediates in the biosynthesis of flavonoids, which are substances widespread in plants and with an array of biological activities. Chalcones are also intermediates in the Auwers synthesis of flavones.
Chemical properties
light yellow powder
The Uses of Chalcone
Chalcone is used in the preparation of pharmacologically-interesting heterocyclic systems like pyrazolines and pyrimidines. It also inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase.
The Uses of Chalcone
1,3-Diphenyl-2-propenone was used in the preparation of pharmacologically-interesting heterocyclic systems like pyrazolines and pyrimidines.
Definition
ChEBI: A member of the class of chalcones that is acetophenone in which one of the methyl hydrogens has been replaced by a benzylidene group.
Preparation
Chalcone is an aromatic ketone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones.
Chalcones can be prepared by an aldol condensation between a benzaldehyde and an acetophenone in the presence of sodium hydroxide as a catalyst.
This reaction has been found to work without any solvent at all - a solid-state reaction. The reaction between substituted benzaldehydes and acetophenones has been used to demonstrate green chemistry in undergraduate chemistry education.In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C).
Biological Activity
1,3-Diphenyl-2-propenone (chalcone) inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. It is an inhibitor of Plasmodium falciparum cyclin-dependent protein kinases.
Biochem/physiol Actions
1,3-Diphenyl-2-propenone (chalcone) inhibits the proliferation of human breast cancer cell lines, MCF-7 and MDA-MB-231 by inducing apoptosis and blocking cell cycle progression in the G2/M phase. It is an inhibitor of Plasmodium falciparum cyclin-dependent protein kinases.
Safety Profile
Poison by intravenous route. See also KETONES. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise it from EtOH by warming to 50o (about 5mL/g), iso-octane, or toluene/pet ether, or recrystallise it from MeOH, and then twice from hexane. SKIN IRRITANT. [Beilstein 7 IV 1658.]
Properties of Chalcone
| Melting point: | 55-59 °C |
| Boiling point: | 208 °C25 mm Hg(lit.) |
| Density | d462 1.0712 |
| refractive index | nD62 1.6458 |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | dioxane: soluble1g/10 mL, clear, colorless |
| form | Granular Powder |
| color | Yellow |
| Merck | 14,2037 |
| BRN | 1210466 |
| CAS DataBase Reference | 94-41-7(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Propen-1-one, 1,3-diphenyl- (94-41-7) |
Safety information for Chalcone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chalcone
Chalcone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

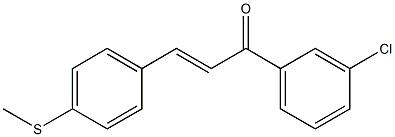
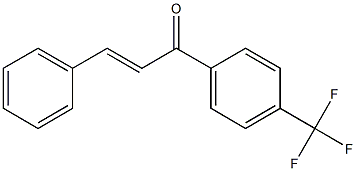
![2-Chloro-α-phenyl-4'-[2-(1-pyrrolidinyl)ethoxy]chalcone](https://img.chemicalbook.in/CAS/20211123/GIF/24845-21-4.gif)
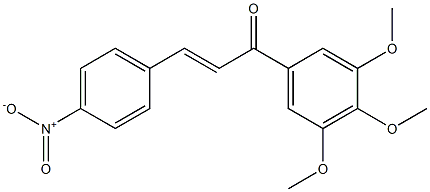
You may like
-
 94-41-7 Chalcone; 1,3- Diphenyl -2- Propenane 98%View Details
94-41-7 Chalcone; 1,3- Diphenyl -2- Propenane 98%View Details
94-41-7 -
 Chalcone 98%View Details
Chalcone 98%View Details
94-41-7 -
 Benzalacetophenone 94-41-7 98%View Details
Benzalacetophenone 94-41-7 98%View Details
94-41-7 -
 Chalcone CAS 94-41-7View Details
Chalcone CAS 94-41-7View Details
94-41-7 -
 Benzalacetophenone (Chalcone) pure CAS 94-41-7View Details
Benzalacetophenone (Chalcone) pure CAS 94-41-7View Details
94-41-7 -
 Chalcone, 98% CAS 94-41-7View Details
Chalcone, 98% CAS 94-41-7View Details
94-41-7 -
 Chalcone 98% (GC) CAS 94-41-7View Details
Chalcone 98% (GC) CAS 94-41-7View Details
94-41-7 -
 ChalconeView Details
ChalconeView Details
94-41-7
