CEPHALEXIN MONOHYDRATE
- CAS NO.:23325-78-2
- Empirical Formula: C16H19N3O5S
- Molecular Weight: 365.4
- MDL number: MFCD00167148
- EINECS: 629-748-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24

What is CEPHALEXIN MONOHYDRATE?
Description
Cephalexin was first synthesized in 1967 by Glaxo Research Laboratories and first produced on an industrial scale by Eli Lilly & Co. in the same year. It is a deacetoxylated derivative of cephaloglycin that is not metabolized in vivo. When administered orally, it shows a much higher serum concentration and much lower tendency to induce diarrhea than cephaloglycin. Cephalexin has been used widely and is the most popular orally active antibiotic in the world for treatment of respiratory tract, urinary tract, surgical, ear and nose, and other infections caused by Staphylococcus, Streptococcus, Escherichia coli, Klebsiella, Enterobacter, and Proteus.
Chemical properties
White to Off-White Solid
Originator
Ceporex,Glaxo,UK,1970
The Uses of CEPHALEXIN MONOHYDRATE
Cephalosporin antibacterial.
The Uses of CEPHALEXIN MONOHYDRATE
A semisynthetic cephalorsporin antibiotic.
The Uses of CEPHALEXIN MONOHYDRATE
Semi-synthetic cephalosporin antibiotic.
Definition
ChEBI: The hydrate of cephalexin.
Manufacturing Process
To a 1 liter flask containing dimethylformamide at 0°C, was added 24.8 g
sodium N-(2-methoxycarbonyl-1-methylvinyl)-D-α-phenylglycine (prepared
from sodium D-α-phenylglycine and methyl acetoacetate). The mixture was
cooled to -40°C and methyl chloroformate (7.5 ml) and dimethylbenzylamine
(0.26 ml) added. After stirring for 25 minutes, p-nitrobenzyl 7-
aminodesacetoxycephalosporanate (32.8 g) in the form of its hydrochloride
salt was added, followed by triethylamine (12.1 ml) and dimethylformamide
(140 ml) over a period of 20 minutes. The reaction mixture was stirred for 2
hours at -25°C to -35°C, then warmed to 0°C and water (32 ml) added. To
the resultant solution, hydrochloric acid (54 ml) was added followed by zinc
(21.8 g) in portions over a period of 5 minutes, the temperature being
maintained at 5°C to 10°C. Further hydrochloric acid (35 ml) was added and
the solution stirred at 15°C to 20°C for 7 hours.
The pH was adjusted to 3.3 with triethylamine and
semicarbazidehydrochloride (9.5 g) added. The mixture was brought back to
pH 3 with further triethylamine, then stirred for 30 minutes at pH 3. The
resultant mixture was adjusted slowly over 4 hours to pH 6.8 by addition of
triethylamine, seeding being carried out when pH 4.5 was reached. The precipitated cephalexin was filtered off, washed with dimethylformamide (200
ml) and the cephalexin recovered, yield 75%.
brand name
Keflex (Panixine (Ranbaxy).
Therapeutic Function
Antibiotic
Properties of CEPHALEXIN MONOHYDRATE
| Melting point: | >161°C (dec.) |
| refractive index | 154 ° (C=0.5, H2O) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | NH4OH 1 M: 50 mg/mL, clear, yellow |
| form | solid |
| color | White to Light Beige |
| Water Solubility | 13.5g/L(25 ºC) |
| Merck | 14,1974 |
| BRN | 965503 |
| Stability: | Unstable in Solution |
| CAS DataBase Reference | 23325-78-2(CAS DataBase Reference) |
Safety information for CEPHALEXIN MONOHYDRATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CEPHALEXIN MONOHYDRATE
| InChIKey | AVGYWQBCYZHHPN-KJTIIWGRSA-N |
CEPHALEXIN MONOHYDRATE manufacturer
Ralington Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
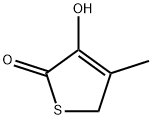
![(6R-trans)-7-[(2,2-diMethyl-1-oxopropyl)aMino]-3-Methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-](https://img.chemicalbook.in/CAS/GIF/146794-70-9.gif)
![[6R-[6α,7β(R*)]]-7-[(AMinophenylacetyl)aMino]-3-Methylene-8-oxo-5-thia-1-azabicyclo[4.2.0]octane-2-carboxylic Acid SodiuM Salt](https://img.chemicalbook.in/CAS/GIF/37050-97-8.gif)
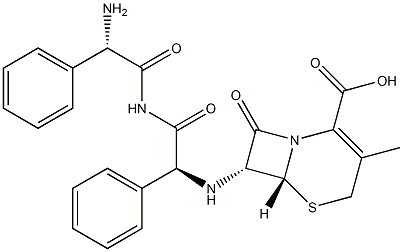
![[6R-[6α,7β(R*)]]-7-[(AMinophenylacetyl)aMino]-3-Methyl-8-oxo-5-thia-1-azabicyclo[4.2.0](https://img.chemicalbook.in/CAS/20150408/GIF/79750-46-2.gif)
You may like
-
 23325-78-2 Cephalexin monohydrate 98%View Details
23325-78-2 Cephalexin monohydrate 98%View Details
23325-78-2 -
 Cephalexin monohydrate 23325-78-2 99%View Details
Cephalexin monohydrate 23325-78-2 99%View Details
23325-78-2 -
 Cephalexin monohydrate CAS 23325-78-2View Details
Cephalexin monohydrate CAS 23325-78-2View Details
23325-78-2 -
 Cephalexin CAS 23325-78-2View Details
Cephalexin CAS 23325-78-2View Details
23325-78-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1