Cemandil sodium salt
Synonym(s):Cefamandole formate sodium salt
- CAS NO.:42540-40-9
- Empirical Formula: C19H17N6NaO6S2
- Molecular Weight: 512.49
- MDL number: MFCD00864877
- EINECS: 255-877-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:11
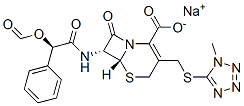
What is Cemandil sodium salt?
Chemical properties
White Solid
Originator
Mandokef,Lilly,W. Germany,1977
The Uses of Cemandil sodium salt
Cephalosporin antibacterial.
The Uses of Cemandil sodium salt
A second generation cephalosporin antibiotic.
What are the applications of Application
Cefamandole nafate is a compound used to study the effects of expression and inhibition of PBP 2A
Definition
ChEBI: A cephalosporin prodrug having (R)-O-formylmandelamido and N-methylthiotetrazole side-groups.
Manufacturing Process
To 21.6 kg (17.8 l) of 98% formic acid was added 1.14 kg (7.5 mols) of D-(-)-
mandelic acid and the reaction mixture was heated for 4 hours at 70°C with
stirring. The excess formic acid was evaporated off in vacuo and the residual
syrup was dissolved in 6 l of benzene. The solution was washed twice with 6 l
portions of water and was dried over magnesium sulfate. The drying agent
was filtered and washed with 1.5 l of benzene, the washes being added to the
filtrate. The dried filtrate was evaporated in vacuo to obtain the D-(-)-
mandelic acid formate ether as a syrup. The product can be crystallized from
cyclohexane to yield material melting at about 55°C to 58°C.
The mandelic acid formate ester obtained as a syrup as described above is
stirred for 2 hours with 2.9 kg (~1.75 l) of thionyl chloride at a temperature
of about 70°C. The excess thionyl chloride is removed by evaporation and the
residual green solution is vacuum distilled. The product, O-formyl mandeloyl
chloride, distills over at 127°C to 130°C (15 mm) or at 108°C to 112°C (7
mm).
To 13 l of ethyl acetate were added 85.1 g (2.59 mols) of 7-amino-3-(1-
methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-carboxylic acid and 1,361 g
(10.37 mols) of monotrimethylsilyl acetamide, and the mixture was stirred at
50°C until a clear solution was obtained. The solution was cooled to 20°C and
514 g (2.59 mold of O-formyl mandeloyl chloride was added at a rate such
that the temperature of the reaction solution was maintained between about
20°C to 25% with ice-cooling.
The reaction mixture was stirred for 1.5 hours at about room temperature
after the addition of the mandeloyl chloride was completed. Five liters of
water were then added to the reaction mixture and the diluted mixture was
stirred for about 10 minutes. The organic layer was separated and was
washed twice with water. The combined washes are extracted with 1.5 l of
ethyl acetate and the extract is combined with the washed organic layer. The
whole was dried over magnesium sulfate, filtered and evaporated in vacuo on
a 25°C water bath to yield 1,460 g of product, 7-(D-2-formyloxy-2-
phenylacetamido)-3-(1-methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-
carboxylic acid, as a yellow foam.
The product was dissolved in 5 l of acetone and the solution was mixed with a
solution of 430 g (2.59 mols) of sodium 2-ethylhexanoate in 5.4 l of acetone.
The combined solutions were seeded and stirred in an ice bath for 1.5 hours.
The crystalline precipitate of sodium 7-(D-2-formyloxy-2-phenylacetamido)-3-
(1-methyl-1H-tetrazol-5-ylthiomethyl)-3-cephem-4-carboxylate was filtered
and washed with 5 l of acetone. The crystalline salt was dried overnight in a
vacuum oven at 40°C to yield 1,060 g (80%)of product, melting at 182°C to
184°C.
brand name
Mandol (Lilly).
Therapeutic Function
Antibiotic
Mechanism of action
Cefamandole nafate has a formylated D-mandelic amide moiety at C-7. The formate ester is cleaved rapidly in the host to release the more active cefamandole. The esterification also apparently overcomes the instability of cefamandole when it is stored in dry form. This agent has increased activity against Haemophil us influenzae and some Gram-negative bacilli as compared with the first-generation cephalosporins. Loss of the 5-thio-l-methyl-l-H-tetrazole moiety (referred to sometimes by the acronym MT T) from C-3 is associated with prothrombin deficiency and bleeding problems as well as with an Antabuse-like acute alcohol intolerance. On the other hand, this grouping enhances potency and prevents metabolism by deacetylation. Like the other second-generation cephalosporins, cefamandole is more active against Gram-negative bacteria. The principle clinical use is for lower respiratory tract, skin and skin structures, and bone and joint infections as well as septicemia and urinary tract infections when the organisms are sensitive.
Clinical Use
The D-mandeloyl moiety of Cemandil sodium salt appears toconfer resistance to a few β-lactamases, since some β-lactamase–producing, Gram-negative bacteria (particularlyEnterobacteriaceae) that show resistance to cefazolin andother first-generation cephalosporins are sensitive tocefamandole. Additionally, it is active against some ampicillin-resistant strains of Neisseria and Haemophilus spp.Although resistance to β-lactamases may be a factor in determiningthe sensitivity of individual bacterial strains tocefamandole, an early study indicated that other factors,such as permeability and intrinsic activity, are frequentlymore important. The L-mandeloyl isomer is significantlyless active than the D-isomer.
Properties of Cemandil sodium salt
| Melting point: | 190-193°C |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Freely soluble in water, sparingly soluble in methanol. |
| form | neat |
| pka | 2.6-3.0(at 25℃) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Freely soluble in water. Sparingly soluble in methanol |
| CAS DataBase Reference | 42540-40-9(CAS DataBase Reference) |
Safety information for Cemandil sodium salt
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Cemandil sodium salt
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran








You may like
-
 Cefamandole nafate 95% CAS 42540-40-9View Details
Cefamandole nafate 95% CAS 42540-40-9View Details
42540-40-9 -
 Cefamandole nafate 95.00% CAS 42540-40-9View Details
Cefamandole nafate 95.00% CAS 42540-40-9View Details
42540-40-9 -
 Cefamandole nafate >98% (HPLC) CAS 42540-40-9View Details
Cefamandole nafate >98% (HPLC) CAS 42540-40-9View Details
42540-40-9 -
 Cefamandole nafate CAS 42540-40-9View Details
Cefamandole nafate CAS 42540-40-9View Details
42540-40-9 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0