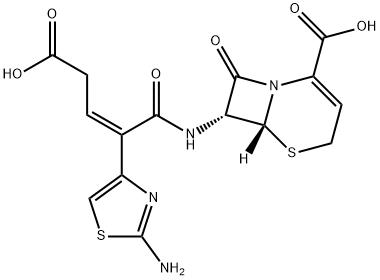
Ceftibuten
- CAS NO.:97519-39-6
- Empirical Formula: C15H14N4O6S2
- Molecular Weight: 410.42
- MDL number: MFCD00864918
- EINECS: 810-182-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:19
What is Ceftibuten?
Absorption
Rapidly absorbed following oral administration.
Toxicity
Overdosage of cephalosporins can cause cerebral irritation leading to convulsions.
Description
Ceftibuten is a new, once daily, orally active cephalosporin introduced as a treatment of Gram-negative bacteria-related urinarylrespiratory tract and gynecological infections. In vitro studies of 359 strains of Gram-negative bacteria demonstrated that ceftibuten was superior to cefaclor and as active or slightly more active than cefixime and cefteram.
Originator
Shionogi (Japan)
The Uses of Ceftibuten
anorexic, antidepressant, inhibitor of 5HT, norepinephrine & dopamine uptake
Background
Ceftibuten is a third-generation cephalosporin antibiotic that is orally-administered. It is typically used to treat acute bacterial exacerbations of chronic bronchitis (ABECB), acute bacterial otitis media, pharyngitis, and tonsilitis.
Indications
Indicated for the treatment of acute bacterial exacerbations of chronic bronchitis (ABECB), acute bacterial otitis media, pharyngitis, and tonsilitis.
Definition
ChEBI: A third-generation cephalosporin antibiotic with a [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino substituent at the 7 position of the cephem skeleton. An orally-administered agent, ceftibuten is used as the dihydrate to tre t urinary-tract and respiratory-tract infections.
Manufacturing Process
The 1st method of synthesis
The 8-oxo-7-phenylacetylamino-5-thia-1-aza-bicyclo[4.2.0]oct-1-ene-2-
carboxylic acid benzhydryl ester is reacted with phosphorus
pentachloride/pyridine reagent in methylene dichloride, and the reaction
mixture is thereafter cooled to -35°C and treated with methanol to produce
hydrochloride of 7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-
carboxylic acid benzhydryl ester. This hydrochloride is reacted with 4-(3-
aminothiophen-2-yl)-5-oxohex-3-enoic acid 3-methylbut-2-enyl ester. Then 7-
[2-(2-benzoylamino-thiazol-5-yl)(3-tert-butyl-4,4-dimethylpent-2-
enoxycarbonyl)-pent-2-enoylamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-
ene-2-carboxylic acid synthesized is reacted with aluminum chloride in anisole
and diluted hydrochloric acid and then with dimethylmalonate to give 5-thia-
1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(((2Z)-2-(2-amino-4-
thiazolyl)-4-carboxy-1-oxo-2-butenyl)amino)-8-oxo-, (6R,7R)- (Ceftibuten).
The 2st method of synthesis
Formulation of the diphenylmethyl thiazoleacetate with ethyl formate leads to
2-(2-aminothiazol-5-yl)-3-hydroxyacrylic acid benzhydryl ester. Condensation
of 2-(2-aminothiazol-5-yl)-3-hydroxyacrylic acid benzhydryl ester with the
phosphorane from benzyl 2-triphenylphosphonium acetate leads to the 2-(2-
aminothiazol-5-ylmethylene)succinic acid 1-benzhydryl ester 4-benzyl ester.
Exposure of this ester to trifluoroacetic acid selectively cleaves the
diphenylmethyl group over the benzyl ester to give 2-(2-aminothiazol-5-
ylmethylene)succinic acid 4-benzyl ester. Condensation of the acid with free
amino group in the desmethyl cephalosporin affords the amide of 7-[3-(2-
aminothiazol-5-yl)-2-benzoylcarbonylmethylacryloylamino)-8-oxo-5-thia-1-
azabicyclo[4.2.1]oct-2-ene-2-carboxylic acid benzyl ester. The remaining
benzyl ester protecting groups are removed by means of aluminum chloride to
afford 7-[3-(2-aminothiazol-5-yl)-2-benzoylcarbonylmethylacryloylamino)-8-
oxo-5-thia-1-azabicyclo[4.2.1]oct-2-ene-2-carboxylic acid or ceftibuten
brand name
Cedax (Schering);Seftem.
Therapeutic Function
Antibiotic
Antimicrobial activity
A semisynthetic cephalosporin formulated as the dihydrate
for oral administration.
It exhibits good activity against many Gram-negative bacilli,
but its activity against Gram-positive cocci is very poor. It
is stable to hydrolysis by the common plasmid-mediated
β-lactamases, but not derepressed chromosomal enzymes .
It is rapidly and almost completely absorbed by mouth and
is excreted in the urine with a half-life of 1.5–3 h. An oral dose
of 400 mg achieves a peak plasma concentration of around
15 mg/L. Binding to plasma proteins is 65–77%.
Side effects mostly consist of mild gastrointestinal symptoms
and mild liver function test changes. Clinical trials have
mainly been conducted in urinary tract and respiratory tract
infections which, despite the poor in-vitro activity against
Str. pneumoniae, have shown ceftibuten to be as efficacious as
comparator agents.
General Description
Chemical structure: ?-lactam
Biochem/physiol Actions
Ceftibuten is a third generation cephalosporin antibiotic
Pharmacokinetics
Ceftibuten is an antibiotic with bactericidal actions.
Pharmacokinetics
Ceftibuten is highly (75–90%) absorbed on oral administration, but this is decreased significantly by food. Being lipophilic and acidic, it is significantly (65%) serum protein bound. Some isomerization of the geometry of the olefinic linkage appears to take place in vivo before excretion.
Clinical Use
Ceftibuten (Cedax) is a recently introduced, chemicallynovel analog of the oximino cephalosporins in which anolefinic methylene group (C=CHCH2-) with Z stereochemistryhas replaced the syn oximino (CBNO-) group.
This isosteric replacement yields a compound that retainsresistance to hydrolysis catalyzed by many β-lactamases,has enhanced chemical stability, and is orally active. Oralabsorption is rapid and nearly complete. It has the highestoral bioavailability of the third-generation cephalosporins.Ceftibuten is excreted largely unchanged in the urine andhas a half-life of about 2.5 hours. Plasma protein binding ofthis cephalosporin is estimated to be 63%.
Ceftibuten possesses excellent potency against mostmembers of the Enterobacteriaceae family, H. influenzae,Neisseria spp., and M. catarrhalis. It is not active against S.aureus or P. aeruginosa and exhibits modest antistreptococcal activity. Ceftibuten is recommended in the managementof community-acquired respiratory tract, urinary tract, andgynecological infections.
Metabolism
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer is approximately 1/8 as antimicrobially potent as the cis-isomer.
Properties of Ceftibuten
| Boiling point: | 966℃ |
| Density | 1.75±0.1 g/cm3(Predicted) |
| RTECS | XI0367220 |
| Flash point: | >110°(230°F) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Soluble in aqueous solutions. Also soluble in DMSO |
| pka | 2.99±0.50(Predicted) |
| form | powder |
| color | white to beige |
Safety information for Ceftibuten
Computed Descriptors for Ceftibuten
Ceftibuten manufacturer
Nectar Lifesciences Ltd
Orchid Pharma Ltd. (Formerly Known as Orchid Chemicals and Pharmaceuticals Ltd.)
Suvan LifeSciences (formerly Sansh Biotech Pvt Ltd)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

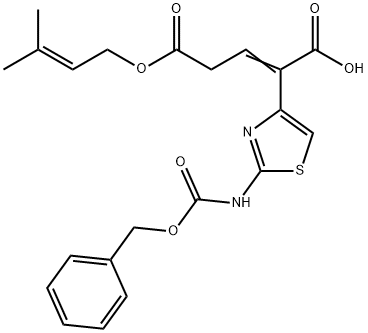


![7-Amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid diphenylmethyl ester](https://img.chemicalbook.in/CAS/GIF/36923-21-4.gif)

You may like
-
 97519-39-6 Ceftibuten 98%View Details
97519-39-6 Ceftibuten 98%View Details
97519-39-6 -
 97519-39-6 99%View Details
97519-39-6 99%View Details
97519-39-6 -
 Ceftibuten 98%View Details
Ceftibuten 98%View Details
97519-39-6 -
 97519-39-6 Ceftibuten 99%View Details
97519-39-6 Ceftibuten 99%View Details
97519-39-6 -
 Ceftibuten hydrate CAS 97519-39-6View Details
Ceftibuten hydrate CAS 97519-39-6View Details
97519-39-6 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0