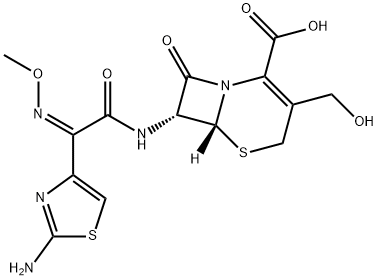
Cefetamet hydrochloride
- CAS NO.:65052-63-3
- Empirical Formula: C14H15N5O5S2
- Molecular Weight: 397.43
- MDL number: MFCD00864836
- EINECS: 675-838-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 20:05:46
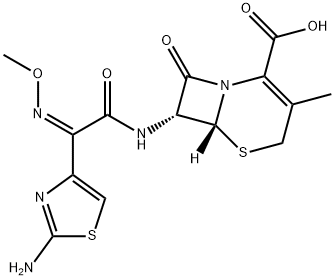
What is Cefetamet hydrochloride?
Description
Ceftazidime hydrochloride is an oral third-generation cephalosporin, which has the same antibacterial activity against gram-positive bacteria as cefixime, and is superior to cefaclor, especially against Streptococcus pneumoniae and Streptococcus pyogenes. It has obvious antibacterial activity against a variety of gram-negative bacteria such as Escherichia coli, Proteus, Klebsiella pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae, especially against Serratia, indole-positive Proteus, Enterobacter The antibacterial activity of Citrobacter and Citrobacter was significantly enhanced.
The Uses of Cefetamet hydrochloride
Cefetamet is a third-generation cephalosporin antibiotic and the active agent of Cefeamet pivoxil (C242780) after hydrolysis.
What are the applications of Application
Cefetamet acid is a third-generation cephalosporin antibiotic
Definition
ChEBI: Cefetamet is a cephalosporin compound having methyl and [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side-groups; a cephalosporin antibiotic active against Neisseria gonorrhoeae.
Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use as the
prodrug ester, cefetamet pivoxyl. It is less active than cefaclor
and cefadroxil against Staph. aureus, but as active against
streptococci and more active against enterobacteria, H. influenzae
and N. gonorrhoeae, including β-lactamase-producing
strains. L. monocytogenes, C. difficile, Sten. maltophilia and Burk.
cepacia are all resistant. It is resistant to hydrolysis by common
plasmid-mediated enzymes.
The absolute bioavailability is about 50%. The plasma peak
is delayed by food. Binding to plasma protein is about 20%.
The volume of distribution approximates to the extracellular
water. It is excreted into urine with a half-life of 2–2.5 h,
principally in the glomerular filtrate. Elimination is linearly
related to creatinine clearance. Side effects and uses are similar
to those of other group 5 cephalosporins.
Properties of Cefetamet hydrochloride
| Melting point: | >178°C (dec.) |
| Density | 1.83±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 3.11±0.50(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 65052-63-3(CAS DataBase Reference) |
Safety information for Cefetamet hydrochloride
Computed Descriptors for Cefetamet hydrochloride
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
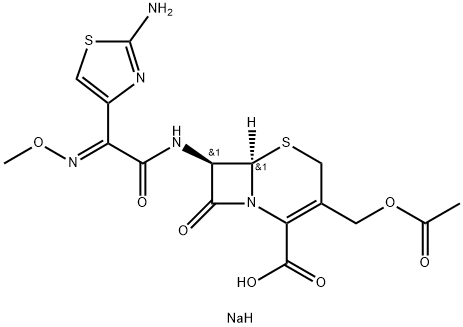
![[6R-[6alpha,7beta(Z)]]-3-(acetoxymethyl)-7-[[2-(formylamino)thiazol-4-yl](methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](https://img.chemicalbook.in/CAS/GIF/66403-32-5.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1