CARAZOLOL HCL
Synonym(s):1-(Carbazol-4-yloxy)-3-(isopropylamino)-2-propanol;4-(2-Hydroxy-3-isopropylamino-propoxy)carbazole
- CAS NO.:57775-29-8
- Empirical Formula: C18H22N2O2
- Molecular Weight: 298.38
- MDL number: MFCD00864588
- EINECS: 260-945-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
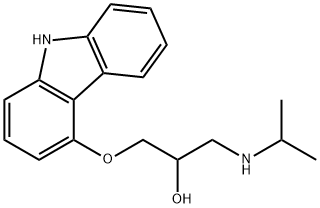
What is CARAZOLOL HCL?
Chemical properties
Yellowish solid
Originator
Conducton,Klinge,W. Germany,1980
The Uses of CARAZOLOL HCL
Carazolol is a promising high-affinity beta-adrenergic receptor ligand for the noninvasive determination of beta receptor status using PET
The Uses of CARAZOLOL HCL
Carazolol is a high-affinity, lipophilic, non-selective ligand of the β-adrenergic receptors (Kis = 0.114 and 0.4 nM for β2- and β3-adrenoceptors, respectively). It acts as a receptor antagonist (beta blocker) or inverse agonist for β1 and β2 receptors but functions as an agonist at the β3-adrenergic receptor.
What are the applications of Application
Carazolol is A high affinity β-adrenergic receptor antagonist
Definition
ChEBI: Carazolol is a member of carbazoles.
Manufacturing Process
The 4-(2,3-epoxypropoxy)carbazole used as starting material is prepared as
follows. A solution of 16.3 g 4-hydroxycarbazole in a mixture of 190 ml dioxan
and 98 ml 1 N sodium hydroxide is, after the addition of 66 ml
epichlorohydrin, stirred for 2 hours at 40°C to 45°C. The reaction mixture is then diluted with water and shaken out with methylene chloride. The
methylene chloride phase is washed with water, dried over anhydrous sodium
sulfate and evaporated. There are obtained 16.8 g 4-(2,3-
epoxypropoxy)carbazole.
A solution of 3.5 g 4-(2,3-epoxypropoxy)carbazole in 50 ml absolute alcohol is
mixed with 30 ml isopropylamine and heated for 3 hours under reflux. When
the reaction is finished, the reaction mixture is evaporated to dryness. The
residue obtained is taken up in methylene chloride and chromatographed over
an aluminum oxide column (300 g basic aluminum oxide, activity stage IV;
eluent methylene chloride). The eluted fractions are evaporated and the
residue is dissolved in methanol and acidified with 2 N ethereal hydrochloric
acid.
The precipitate obtained is filtered off and recrystallized from methanol. There
are obtained 3.1 g (62% of theory) 4-(3-isopropylamino-2-hydroxypropoxy)
carbazole hydrochloride; MP 234°C to 235°C.
Therapeutic Function
Beta-adrenergic blocker
Biological Activity
carazolol is a high-affinity, lipophilic, and non-selective ligand of the β-adrenergic receptors [1,2].β-adrenergic receptors have been involved in mediating the physiological responses of the catecholamines, epinephrine and norepinephrine and modulating a myriad of physiological functions, such as relaxation of smooth muscle, chronotropic and inotropic cardiac responses, and lipolysis in adipose tissue. the β-adrenergic receptors exist in nearly all mammalian tissues. until now, three β-adrenergic receptors have been identified, β1-, β2- and β3-adrenergic receptors [3].in cho cells expressing the human β3-adrenoceptor, the ki value of carazolol was 2.0 ± 0.2 and the ic50 was 11.3 ± 1.2 nm. carazolol exhibited an ecs0 of 25 nm and behaved as a full agonist (intrinsic activity = 0.97) towards the murine β3-adrenoceptor. in murine 3t3-f442a-derived adipocytes express the β3-adrenoceptor, carazolol stimulated lipolysis [1]. carazolol bound to cortical β-receptors with a kd value of 0.15 nm. carazolol showed equal displacements constants when binding with calf cerebral cortex (which contained mainly β1 receptors) and calf cerebellum (which contained mainly β2 receptors), indicating that carazolol bound with equal affinity to β1 and β2 receptors [2].
References
[1] méjean a, guillaume j l, strosberg a d. carazolol: a potent, selective β3-adrenoceptor agonist[j]. european journal of pharmacology: molecular pharmacology, 1995, 291(3): 359-366.
[2] innis r b, corrêa f m a, snyder s h. carazolol, an extremely potent β-adrenergic blocker: binding to β-receptors in brain membranes [j]. life sciences, 1979, 24(24): 2255-2264.
[3] pellegrino s m, lee n h, fraser c m. β-adrenergic receptors[j]. biomembranes: a multi-volume treatise, 1996, 2: 245-279.
Properties of CARAZOLOL HCL
| Melting point: | 133-137 °C |
| storage temp. | 2-8°C(protect from light) |
| solubility | insoluble in H2O; ≥14.8 mg/mL in DMSO; ≥3.9 mg/mL in EtOH |
| form | neat |
| form | Solid |
| color | White to Almost white |
| λmax | 332nm(Cabonate butter pH 9.3)(lit.) |
| Merck | 14,1778 |
| BRN | 3620576 |
| CAS DataBase Reference | 57775-29-8(CAS DataBase Reference) |
Safety information for CARAZOLOL HCL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CARAZOLOL HCL
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Carazolol 95% CAS 57775-29-8View Details
Carazolol 95% CAS 57775-29-8View Details
57775-29-8 -
 Carazolol CAS 57775-29-8View Details
Carazolol CAS 57775-29-8View Details
57775-29-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4