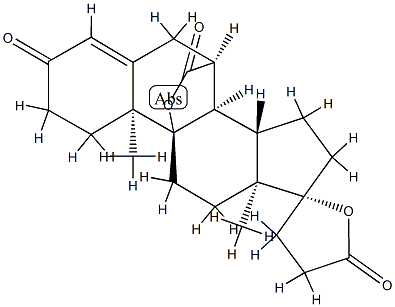
Canrenone
Synonym(s):(17α)-17-Hydroxy-3-oxopregna-4,6-diene-21-carboxylic acid γ-lactone;Canrenone;NSC 261713
- CAS NO.:976-71-6
- Empirical Formula: C22H28O3
- Molecular Weight: 340.46
- MDL number: MFCD00071735
- EINECS: 213-554-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
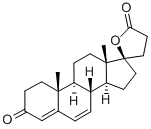
What is Canrenone?
Chemical properties
Pale yellow to pale Green Solid
Originator
Spiroctan,Boehringer Mannheim,Switz.,1968
The Uses of Canrenone
Aldosterone antagonist. Diuretic
The Uses of Canrenone
antiinflammatory, glucocorticoid
The Uses of Canrenone
Inhibits aldosterone biosynthesis and blocks ouabain effects
What are the applications of Application
Canrenone is an inhibitor of aldosterone biosynthesis and ouabain
Definition
ChEBI: Canrenone is a steroid lactone.
Manufacturing Process
The lactone is prepared as follows: A solution of 5 parts of 17α-carboxyethyl- 17β-hydroxyandrost-4-en-3-one lactone and 5 parts of chloranil in 400 parts of xylene containing a trace of p-toluenesulfonic acid is heated at the boiling point of the solvent under reflux overnight. The solution is then cooled and filtered through approximately 200 parts of silica gel. The gel is successively washed with 5%, 10%, and 15% ethyl acetate-benzene solutions, and the washings comprising 15% ethyl acetate are thereupon purified by chromatography on a further quantity of silica gel, using benzene and ethyl acetate as developing solvents. From the 15% ethyl acetate eluate there is obtained pure 17α-carboxyethyl-17β-hydroxyandrost-4,6-dien-3-one lactone, melting at 148° to 151°C. The product solidifies above this melting point and melts again at 165°C.
brand name
Luvion.
Therapeutic Function
Aldosterone antagonist, Diuretic
World Health Organization (WHO)
Canrenone, which has aldosterone antagonist activity, is a major metabolite of spironolactone and the major metabolite of potassium canrenoate. See WHO comments for potassium canrenoate and spironolactone.
Flammability and Explosibility
Non flammable
Biological Activity
Mineralocorticoid receptor antagonist. Active metabolite of spironolactone ((7a,17a)-7-(Acetylthio)-17-hydroxy-3-oxopregn-4-ene-21-carboxylic acid g-lactone ).
Biochem/physiol Actions
Canrenone is a mineralocorticoid (aldosterone) inhibitor.
Properties of Canrenone
| Melting point: | 158-1600C |
| Boiling point: | 416.25°C (rough estimate) |
| alpha | D +24.5° (chloroform) |
| Density | 1.1236 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | room temp |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +17 to +24°, c = 1 in chloroform-d |
| Water Solubility | 272.4ug/L(25 ºC) |
| InChI | InChI=1/C22H28O3/c1-20-9-5-15(23)13-14(20)3-4-16-17(20)6-10-21(2)18(16)7-11-22(21)12-8-19(24)25-22/h3-4,13,16-18H,5-12H2,1-2H3/t16-,17+,18+,20+,21+,22-/s3 |
| CAS DataBase Reference | 976-71-6 |
| NIST Chemistry Reference | Canrenone(976-71-6) |
Safety information for Canrenone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Canrenone
| InChIKey | UJVLDDZCTMKXJK-MUWITHSMNA-N |
| SMILES | [C@@]12(CCC(=O)O1)CC[C@@]1([H])[C@]3([H])C=CC4=CC(=O)CC[C@]4(C)[C@@]3([H])CC[C@]21C |&1:0,8,10,20,22,26,r| |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 976-71-6 Spiranolactone Impurity F 98%View Details
976-71-6 Spiranolactone Impurity F 98%View Details
976-71-6 -
 Canrenone 98% CAS 976-71-6View Details
Canrenone 98% CAS 976-71-6View Details
976-71-6 -
 Spironolactone Related Compound A CAS 976-71-6View Details
Spironolactone Related Compound A CAS 976-71-6View Details
976-71-6 -
 Spironolactone Related Compound A CAS 976-71-6View Details
Spironolactone Related Compound A CAS 976-71-6View Details
976-71-6 -
 Canrenone CAS 976-71-6View Details
Canrenone CAS 976-71-6View Details
976-71-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
