Calcium tungstate
Synonym(s):CALC;calcium binding atopy-related autoantigen 1;Calcium-binding atopy-related autoantigen 1;CBARA1;EFHA3
- CAS NO.:7790-75-2
- Empirical Formula: CaO4W
- Molecular Weight: 287.92
- MDL number: MFCD00010913
- EINECS: 232-219-4
- Update Date: 2025-01-27 09:38:02
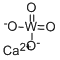
What is Calcium tungstate?
Chemical properties
-325 mesh, 10μm or less 99.9% pure; occurs in nature as mineral scheelite; white tetr powder(s), a=0.524 nm, c=1.138 nm; can be prepared by heating tungstic acid and CaO or CaCO3; used in tumor treatment and in luminous paint [KIR83] [STR93] [MER06]
Large orange octahedral scheelite, Ca(WO4), crystal to 4.5 cm on muscovite, KAl2(AlSi3O10)(OH)2. Pingwu Co., Mianyang pref.,
Sichuan prov., China.
The Uses of Calcium tungstate
Calcium tungstate can be ued for preparing screens for x-ray observations and photographs; in luminous paints; in scintillation counters.
The Uses of Calcium tungstate
Calcium tungsten oxide is used as a luminophore.
Production Methods
A single crystal is grown from melt by the Czochralski method. Rare earth ions are easy to incorporate into the crystal during growth. Fluorescent films are deposited on a glass substrate through vacuum evaporation using a W filament heated at 2000℃ for about 3 min, followed by thermal annealing in oxygen at 550℃ for 2 h. The film becomes crystalline through this thermal annealing.
What are the applications of Application
Calcium tungstate is well known as the phosphor that emits light in λ: 310–700 nm under the excitation light in λ: 220–300 nm. The peak wavelength is positioned at 440 nm, causing it to give off a blue color.
General Description
Calcium tungstate (CaWO4) is an optical material, which can be used as a laser host material for a variety of electronic applications. It has a scheelite structure with luminescence, and thermo-luminescence properties.
Structure and conformation
The space lattice of CaWO4 belongs to the tetragonal system, and its Scheelite structure has lattice constants of a=0.524 nm and c=1.138 nm. The space group is I41/a, and there are four formula units per unit cell.
Properties of Calcium tungstate
| Melting point: | 1620°C |
| Density | 6.06 g/mL at 25 °C (lit.) |
| storage temp. | -20°C |
| solubility | Insoluble in H{2}O. Decomposes by hot HCl, HNO{3} |
| form | Powder |
| color | white |
| Specific Gravity | 6.062 |
| Water Solubility | insoluble H2O; decomposed by hot HCl, HNO3 [MER06] |
| Merck | 14,1712 |
| Solubility Product Constant (Ksp) | pKsp: 8.06 |
| Exposure limits | ACGIH: TWA 3 mg/m3 NIOSH: TWA 5 mg/m3; STEL 10 mg/m3 |
| CAS DataBase Reference | 7790-75-2(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium tungsten oxide (CaWO4) (7790-75-2) |
Safety information for Calcium tungstate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Calcium tungstate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 CALCIUM TUNGSTATE 99%View Details
CALCIUM TUNGSTATE 99%View Details -
 7790-75-2 98%View Details
7790-75-2 98%View Details
7790-75-2 -
 Calcium tungsten oxide CAS 7790-75-2View Details
Calcium tungsten oxide CAS 7790-75-2View Details
7790-75-2 -
 Calcium tungsten oxide CAS 7790-75-2View Details
Calcium tungsten oxide CAS 7790-75-2View Details
7790-75-2 -
 Calcium tungsten oxide CAS 7790-75-2View Details
Calcium tungsten oxide CAS 7790-75-2View Details
7790-75-2 -
 Calcium tungsten oxide CAS 7790-75-2View Details
Calcium tungsten oxide CAS 7790-75-2View Details
7790-75-2 -
 Calcium tungsten oxide CAS 7790-75-2View Details
Calcium tungsten oxide CAS 7790-75-2View Details
7790-75-2 -
 Calcium tungstate CAS 7790-75-2View Details
Calcium tungstate CAS 7790-75-2View Details
7790-75-2
