Calcium Alginate
Synonym(s):Calcium alginate
- CAS NO.:9005-35-0
- Empirical Formula: C18H24CaO19
- Molecular Weight: 1170.93
- MDL number: MFCD00143567
- EINECS: 234-394-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:24
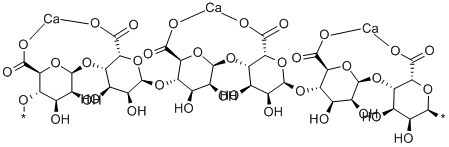
What is Calcium Alginate?
Description
Calcium Alginate is the calcium salt of alginic acid, a natural polyuronide constituent of certain brown algae. Calcium alginate is prepared by the neutralization of purified alginic acid with appropriate pH control agents, or from sodium alginate by metathesis with appropriate calcium salts.
Alginate, a colloidal polyuronic acid structural molecule capable of gelation, is used in the preparation of colloidal biodegradable structures such as gels, biofilms, beads, nanoparticles, and microcapsules suitable for applications that range from gel based separation technologies to drug delivery and cell preservation.
Calcium alginate has been around for years. More recently, it has been added to wound gels, hydrocolloids, and cleansers. Silver, a known antimicrobial agent, also has been added to many wound products, including calcium alginate. Unadulterated calcium alginate comes in the form of a flat square or mat (in many sizes) or a rope. The ropes can be manipulated to fit easily in wound cavities. Although one of its stated uses is to provide homeostasis, calcium alginate is more commonly thought of as the dressing that can absorb 20 times its weight in exudate, soak up loose debris from the wound bed, provide an optimal environment for healing, and provide a painless dressing change. This study is a reminder that calcium alginate is a valuable tool in our wound care arsenal.
Chemical properties
Calcium alginate is an odorless or almost odorless, tasteless, white to pale yellowish-brown powder or fibers.
The Uses of Calcium Alginate
Alginate is used in many applications and new ones are being found all the time. The uses range from applications in the food industry to wound dressings, medicines and dental impression materials.
- Calcium alginate (the cross-linked polymer) is used in wound dressings. These dressings are particularly useful for slow healing wounds like leg ulcers, which can continue to bleed and weep for a long time. Part of the blood clotting mechanism involves calcium ions and on contact with blood the calcium alginate releases calcium ions in exchange for sodium ions – just as you observed in the experiment above. These extra calcium ions can help the blood to clot and encourage healing. It is easy to remove any excess calcium alginate when the dressing has to be changed.
- Calcium alginate dressings with silver have been found to be safe and effective for use for leg ulcers.
- Calcium alginate dressings are made from salts of alginic acid obtained from algae (Phaeophyceae sp.j found in seaweed. They are known for absorbing excess wound exudate and forming a non-adherent gel, which accelerates wound healing by promoting a moist wound healing environment, facilitating debridement, and helping to prevent trauma to the wound bed and the surrounding skin (Fanucci and Seese, 1991).
- Calcium alginate dressings are used on moderate to heavily exudative wounds during the transition from debridement to repair phase of wound healing (Seymour, 1997, Joël et al., 2002). Dry wounds should not be treated with these dressings because they have no hydrating properties.
The Uses of Calcium Alginate
calcium alginate is used as a visual accent in cosmetics. This is an alginate gel impregnated with an oily core material that can be pigmented or neutral in color. It can also be used to mask odor and adjust product viscosity.
The Uses of Calcium Alginate
Calcium Alginate is the calcium salt of alginic acid which functions as a stabilizer and thickener. the partial obtainment of calcium alg- inate by the reaction of the water-soluble alginate with calcium ions is used to obtain viscosity and gel formation. it is used in icings, imitation pulp, dessert gels, and fabricated fruits.
What are the applications of Application
Alginic acid calcium salt from brown algae is used in the preparation of colloidal biodegradable structures such as gels, biofilms, beads, nanoparticles, and microcapsules
Production Methods
Calcium alginate can be obtained from seaweed, mainly species of
Laminaria.
Solutions of sodium alginate interact with an ionized calcium
salt, resulting in the instantaneous precipitation of insoluble calcium
alginate, which can then be further processed. Introducing varying
proportions of sodium ions during manufacture can produce
products having different absorption rates.
Pharmaceutical Applications
In pharmaceutical formulations, calcium alginate and calciumsodium
alginate have been used as tablet disintegrants. The use of
a high concentration (10%) of calcium-sodium alginate has been
reported to cause slight speckling of tablets.
A range of different types of delivery systems intended for oral
administration have been investigated. These exploit the gelling
properties of calcium alginate. Calcium alginate beads have been
used to prepare floating dosage systems containing amoxicillin, furosemide, meloxicam, and barium sulfate,(10) and as a
means of providing a sustained or controlled-release action for
sulindac, diclofenac, tiaramide, insulin, and ampicillin. The effect of citric acid in prolonging the gastric retention
of calcium alginate floating dosage forms has been reported.
Impregnating meloxicam in calcium alginate beads may reduce the
risk of ulceration and mucosal inflammation following oral
adminstration. The use of calcium alginate beads, reinforced
with chitosan, has been shown to slow the release of verapamil,
and may be useful for the controlled release of protein drugs to the
gastrointestinal tract. The bioadhesive properties, swelling
and drug release of calcium alginate beads have also been
investigated.
A series of studies investigating the production, formulation,) and drug release from calcium alginate matrices for oral
administration have been published. The release of diltiazem
hydrochloride from a polyvinyl alcohol matrix was shown to be
controlled by coating with a calcium alginate membrane; the drug
release profile could be modified by increasing the coating thickness
of the calcium alginate layer. The microencapsulation of live
attenuated Bacillus Calmette–Guerin (BCG) cells within a calcium
alginate matrix has also been reported.
It has been shown that a modified drug release can be obtained
from calcium alginate microcapsules, pellets, and microspheres. When biodegradable bone implants composed of
calcium alginate spheres and containing gentamicin were introduced
into the femur of rats, effective drug levels in bone and soft
tissue were obtained for 30 days and 7 days, respectively. The
incorporation of radioactive particles into calcium alginate gels may
be useful for the localized delivery of radiation therapy to a wide
range of organs and tissues.
Therapeutically, the gelling properties of calcium alginate are
utilized in wound dressings in the treatment of leg ulcers, pressure
sores, and other exuding wounds. These dressings are highly
absorbent and are suitable for moderately or heavily exuding
wounds. Calcium alginate dressings also have hemostatic properties,
with calcium ions being exchanged for sodium ions in the
blood; this stimulates both platelet activation and whole blood
coagulation. A mixed calcium–sodium salt of alginic acid is used as
fibers in dressings or wound packing material.
Sterile powder consisting of a mixture of calcium and sodium
alginates has been used in place of talc in glove powders.
In foods, calcium alginate is used as an emulsifier, thickener, and
stabilizer.
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Calcium alginate is widely used in oral and topical formulations,
and in foods.
In 1974, the WHO set an estimated acceptable daily intake of
calcium alginate of up to 25 mg, as alginic acid, per kilogram bodyweight.
When heated to decomposition, it emits acrid smoke and
irritating fumes.
LD50 (rat, IP): 1.41 g/kg
LD50 (rat, IV): 0.06 g/kg
Metabolism
Not Available
Storage
Calcium alginate can be sterilized by autoclaving at 1158℃ for 30 minutes or by dry heat at 1508℃ for 1 hour. Calcium alginate should be stored in airtight containers.
Incompatibilities
Calcium alginate is incompatible with alkalis and alkali salts. Propranolol hydrochloride has been shown to bind to alginate molecules, suggesting that propranolol and calcium ions share common binding sites in the alginate chains; the formation of the calcium alginate gel structure was impeded in the presence of propranolol molecules.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral tablets). Included in nonparenteral medicines licensed in the UK.
Properties of Calcium Alginate
| Density | 2.1173 g/cm3 |
| FEMA | 2015 | ALGINATES, SODIUM, CALCIUM, AND AMMONIUM |
| storage temp. | room temp |
| solubility | Practically insoluble in chloroform, ethanol, ether,
water, and other organic solvents. Soluble in dilute solutions of
sodium citrate and of sodium bicarbonate and in sodium
chloride solution. Soluble in alkaline solutions or in solutions
of substances that combine with calcium. |
| form | powder |
| Specific Gravity | 1.6 |
| color | White to Gray to Brown |
| Odor | odorless |
| Merck | 14,242 |
| CAS DataBase Reference | 9005-35-0 |
| EPA Substance Registry System | Calcium alginate (9005-35-0) |
Safety information for Calcium Alginate
Computed Descriptors for Calcium Alginate
Calcium Alginate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 9005-35-0 Calcium alginate 99%View Details
9005-35-0 Calcium alginate 99%View Details
9005-35-0 -
 9005-35-0 99%View Details
9005-35-0 99%View Details
9005-35-0 -
 Calcium alginate 98%View Details
Calcium alginate 98%View Details
9005-35-0 -
 Calcium alginate CAS 9005-35-0View Details
Calcium alginate CAS 9005-35-0View Details
9005-35-0 -
 Calcium Alginate CAS 9005-35-0View Details
Calcium Alginate CAS 9005-35-0View Details
9005-35-0 -
 Alginic acid calcium salt from brown algae CAS 9005-35-0View Details
Alginic acid calcium salt from brown algae CAS 9005-35-0View Details
9005-35-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
