BROMOENOL LACTONE
Synonym(s):BEL;E-6-(Bromoethylene)tetrahydro-3-(1-naphthyl)-2H-pyran-2-one
- CAS NO.:88070-98-8
- Empirical Formula: C16H13BrO2
- Molecular Weight: 317.18
- MDL number: MFCD00270871
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 22:22:14
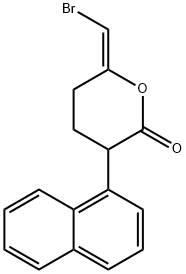
What is BROMOENOL LACTONE?
The Uses of BROMOENOL LACTONE
HELSS (Haloenol lactone suicide substrate, BEL, Bromoenol lactone) is an inhibitor of Ca-independent PLA2 and Mg-dependent PAP.
What are the applications of Application
HELSS (Haloenol lactone suicide substrate, BEL, Bromoenol lactone) is an inhibitor of Ca-independent PLA2 and Mg-dependent PAP
Definition
ChEBI: Bromoenol lactone is a member of naphthalenes.
Biological Activity
bromoenol lactone is a potent and irreversible inhibitor of myocardial cytosolic calcium-independent phospholipase a2 (ipla2) [1].the ipla2 has been involved in stimulus-induced arachidonic acid release and lysophospholipid generation. the catalytic action of ipla2 is responsible for phospholipid remodeling as a housekeeping function. arachidonic acid and lysophospholipid generated by ipla2 act as a signaling molecule in cellular functions, including eicosanoid production, glucose-induced insulin secretion, fas-induced apoptosis, cellular proliferation, membrane traffic in fusion, contribution to myocardial ischemia, and others [2].bel promoted apoptosis in a variety of cell lines, including u937, thp-1, and monomac (human phagocyte), raw264.7 (murine macrophage), jurkat (human t lymphocyte), and gh3 (human pituitary). long term treatment with bel (up to 24 h) increased annexin-v binding to the cell surface and nuclear dna damage. bel induced the proteolysis of procaspase-9 and procaspase-3 and increased cleavage of poly (adp-ribose) polymerase [1]. bel inhibited cellular phosphatidic acid phosphohydrolase (pap) activity in intact p388d1 macrophages with an ic50 of ~8 μm. bel blocked triacylglycerol biosynthesis in p388d1 cells by decreasing diacylglycerol availability [3].
Biochem/physiol Actions
Potent, irreversible inhibitor of calcium-independent phospholipase A2 and of magnesium-dependent phosphatidate phosphohydrolase from P388D macrophages (IC50 = 8?μM); enzyme activated irreversible chymotrypsin inhibitor (Ki = 636 nM).
References
[1] fuentes l, pérez r, nieto m l, et al. bromoenol lactone promotes cell death by a mechanism involving phosphatidate phosphohydrolase-1 rather than calcium-independent phospholipase a2[j]. journal of biological chemistry, 2003, 278(45): 44683-44690.
[2] akiba s, sato t. cellular function of calcium-independent phospholipase a2[j]. biological and pharmaceutical bulletin, 2004, 27(8): 1174-1178.
[3] balsinde j, dennis e a. bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse p388d1 macrophages[j]. journal of biological chemistry, 1996, 271(50): 31937-31941.
Properties of BROMOENOL LACTONE
| Melting point: | 103-106℃ |
| Boiling point: | 467.0±45.0 °C(Predicted) |
| Density | 1.538±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: Dilute in aqueous medium immediately prior to use and store on ice for no more than 12 hours.soluble |
| form | White solid. |
| color | White to yellow |
Safety information for BROMOENOL LACTONE
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for BROMOENOL LACTONE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Bromoenol lactone CAS 88070-98-8View Details
Bromoenol lactone CAS 88070-98-8View Details
88070-98-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
