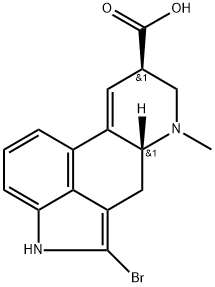
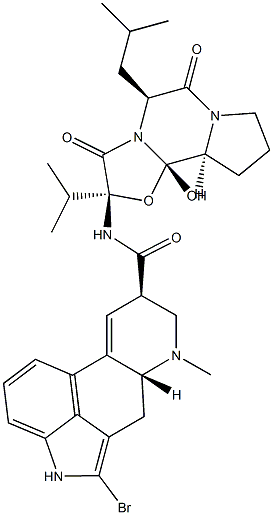
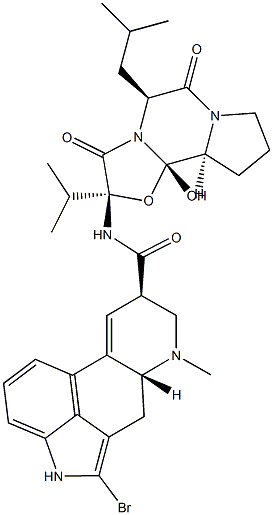
Bromocriptine mesylate
Synonym(s):(+)-2-Bromo-12′-hydroxy-2′-(1-methylethyl)-5′-(2-methylpropyl)ergotaman-3′,6′-18-trione methanesulfonate salt;(+)-Bromocriptine methanesulfonate salt;2-Bromo-α-ergocryptine methanesulfonate salt;Bromocriptine mesylate salt
- CAS NO.:22260-51-1
- Empirical Formula: C33H44BrN5O8S
- Molecular Weight: 750.7
- MDL number: MFCD09028065
- EINECS: 244-881-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
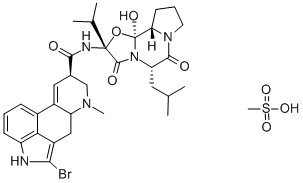
What is Bromocriptine mesylate?
Description
Bromocriptine is a dopamine receptor agonist (Kis = 1,659, 12.2, 12.2, 59.7, and 1,691 nM for dopamine D1, D2, D3, D4, and D5 receptors, respectively). It also binds to the serotonin (5-HT) receptor subtypes 5-HT1A and 5-HT1D (Kis = 12.9 and 10.7 nM, respectively), as well as α1-adrenergic receptors (Kis = 1.12-4.17 nM). Bromocriptine (5 mg/kg) restores locomotor activity, without inducing dyskinesia, in a macaque model of Parkinson’s disease induced by MPTP. Formulations containing bromocriptine have been used in the treatment of Parkinson''s disease, hyperprolactinemia-associated dysfunctions, and acromegaly.
Chemical properties
Solid
Originator
Parlodel,Sandoz,UK,1975
The Uses of Bromocriptine mesylate
Dopamine receptor agonist; derivative of the ergotoxin group of ergot alkaloids. Prolactin inhibitor; antiparkinsonian.
The Uses of Bromocriptine mesylate
2-Bromo-α-ergocryptine methanesulfonate salt has been used:
- as D2 agonist in bird zebra finches
- for Prl secretion inhibitor in mice
- for the inhibition of motility in planaria worm
The Uses of Bromocriptine mesylate
2-Bromo-α-Ergocryptine is a dopamine receptor agonist. 2-Bromo-α-Ergocryptine is a derivative of the ergotoxin group of ergot alkaloids. 2-Bromo-α-Ergocryptine is a prolactin inhibitor; antiparkinsoni an.
What are the applications of Application
Bromocriptine mesylate is a selective D2 and D3 dopamine receptor agonist and NOS1 inhibitor
Definition
ChEBI: Bromocriptine methanesulfonate is a methanesulfonate salt. It has a role as an antiparkinson drug. It contains a bromocriptine.
Manufacturing Process
A solution of 3.4 grams of N-bromosuccinimide in 60 cc of absolute dioxane is
added drop wise in the dark, during the course of 5 minutes, to a stirred
solution, heated to 60°C, of 9.2 grams of ergocryptine in 180 cc of absolute
dioxane. The reaction mixture is stirred at this temperature for 70 minutes
and is concentrated to a syrup-like consistency in a rotary evaporator at a
bath temperature of 50°C. The reaction mixture is subsequently diluted with
300 cc of methylene chloride, is covered with a layer of about 200 cc of a 2 N
sodium carbonate solution in a separating funnel and is shaken thoroughly.
The aqueous phase is extracted thrice with 100 cc amounts of methylene
chloride. The combined organic phases are washed once with 50 cc of water,
are dried over sodium sulfate and the solvent is removed under a vacuum.
The resulting brown foam is chromatographed on a 50-fold quantity of
aluminum oxide of activity II-III with 0.2% ethanol in methylene chloride as
eluant, whereby the compound indicated in the heading is eluted immediately
after a secondary fraction which migrates somewhat more rapidly than the
fractions containing the heading compound. The last fractions to leave the
aluminum oxide contain varying amounts of starting material together with
the heading compound, and may be subjected directly, as mixed fractions, to
an afterbromination in accordance with the method described above. The
fractions containing the pure heading compound are combined and crystallized
from methyl ethyl ketonehopropy1 ether. Melting point 215°-218°C
(decomp.), [α]D
20-195° (c = 1 in methylene chloride).
brand name
Parlodel (Novartis).
Therapeutic Function
Prolactin inhibitor
General Description
Bromocriptine mesylate,(6aR,9R)-5-bromo-N-((2R,5S,10aS,10bS)-10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxooctahydro-2H-oxazolo[3,2-a]pyrrolo[2,1c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate(Parlodel), is a white solid soluble in ethanol and slightly solublein water (pKa’s=6.6 and 15). Bromocriptine is rapidlyabsorbed after oral administration and it has low systemicbioavailability because of its extensive first-pass metabolism.Bromocriptine enters the brain quickly with a half-lifefor uptake into the brain of approximately 0.3 hours; 8% ofthe drug crosses the BBB.The metabolites are excreted primarilyin the bile and feces. The high first-pass hepatic metabolismimplies an increased risk of drug interactions.Concomitant administration with the DA antagonists, metoclopramide,or domperidone may aggravate parkinsoniansymptoms and induce extrapyramidal side effects (EPS).Other drugs that may interact with bromocriptine are highlyplasma protein–bound drugs (e.g., warfarin, increased dyskinesiacaused by bromocriptine); macrolides antibacterials(enhanced dopaminergic effects); and caffeine (elevation inplasma bromocriptine concentrations). The combination oflevodopa/AADC inhibitors with bromocriptine permits a reductionof the dose of levodopa. Thus, the side effects oflevodopa are decreased, resulting in a more continuous stimulationof DA receptors.
Biological Activity
Selective D 2 -like dopamine receptor agonist (K i values are ~ 8, ~ 5, ~ 290, ~ 440 and ~ 450 nM for D 2 , D 3 , D 4 , D 1 and D 5 receptors respectively).
Biochem/physiol Actions
Bromocriptine is an ergot alkaloid and a dopamine D2 receptor agonist. It is prescribed for Parkinson′s disorder, hyperprolactinemia and galactorrhoea. It modulates β cells of the pancreas from insulin hypersecretion and improves the metabolic profile in type 2 diabetes patients with obesity. It also modulates glutamate release by glutamate transporter,GLT-1.
Veterinary Drugs and Treatments
Bromocriptine may potentially be of benefit in treating acromegaly/ pituitary adenomas or pseudopregnancy in a variety of species. However, because of adverse effects, its potential value for treating hyperadrenocorticism in dogs is low. It has been used in dogs for pregnancy termination and pseudopregnancy.
Storage
Room temperature
References
1) Nilsson and Hokfelt (1978), Effect of the dopamine agonist bromocriptine on blood pressure, catecholamines and renin activity in acromegalics at rest, following exercise, and during insulin induced hypoglycemia; Acta Endocrinol., Supp. 216 83 2) Seeman and Van Tol (1994), Dopamine receptor pharmacology; Trends Pharmacol. Sci., 15 264 3) Carvalho et al. (2015), Maternal prolactin inhibition causes changes in leptin at 22- and 30-day old pups; Horm. Metab. Res., 47 528 4) Hubner and Koob (1990), Bromocriptine produces decreases in cocaine self-administration in the rat; Neuropsychopharmacol., 3 101
Properties of Bromocriptine mesylate
| Melting point: | 192-196° (dec) |
| alpha | D20 +95° (c = 1 in methanol-methylene chloride) |
| storage temp. | 2-8°C |
| solubility | H2O: 0.8 mg/mL |
| form | solid |
| pka | 4.90(at 25℃) |
| color | white |
| optical activity | [α]20/D +95°, c = 1 in methanol: methylene chloride (1:1)(lit.) |
| Merck | 13,1400 |
| BRN | 4115238 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months |
| CAS DataBase Reference | 22260-51-1(CAS DataBase Reference) |
Safety information for Bromocriptine mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Bromocriptine mesylate
| InChIKey | NOJMTMIRQRDZMT-JGYCFGIMSA-N |
Bromocriptine mesylate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Bromocriptine mesylate 95% CAS 22260-51-1View Details
Bromocriptine mesylate 95% CAS 22260-51-1View Details
22260-51-1 -
 Bromocriptine mesylate CAS 22260-51-1View Details
Bromocriptine mesylate CAS 22260-51-1View Details
22260-51-1 -
 Bromocriptine Mesylate PowderView Details
Bromocriptine Mesylate PowderView Details
22260-51-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
