Bosutinib Monohydrate
- CAS NO.:918639-08-4
- Empirical Formula: C26H31Cl2N5O4
- Molecular Weight: 548.47
- MDL number: MFCD29920031
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
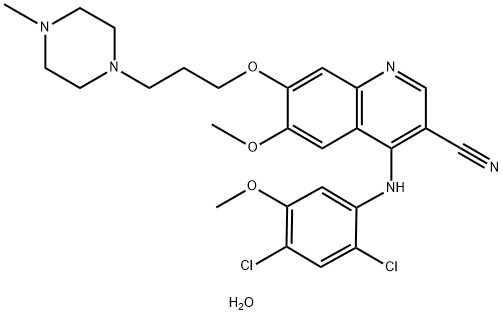
What is Bosutinib Monohydrate?
Definition
ChEBI: A hydrate that is the monohydrate form of anhydrous bosutinib.
Clinical Use
Bosulif ® (Bosutinib hydrate), also known as (SKI-606), is a novel 4-phenylamino-3-quinolinecarbonitrile kinase inhibitor approved for treatment of adults with chronic, accelerated, or blast phase Philadelphia chromosome-positive chronic myeloid leukemia (Ph+CML). Bosutinib is an orally-dosed, dual Src/Abl kinase inhibitor which provides an alternative treatment to patients exhibiting immunity to imatinib and other kinase inhibitors utilized for this treatment. In contrast to competitor tyrosine inhibitors, bosutinib inhibits autophosphorylation of both Srs and Abl kinases, leading to decreased cell growth and apoptosis. Bosutinib was originally developed by Wyeth and continues to be marketed by Pfizer after the merger of Wyeth and Pfizer in 2009.
Synthesis
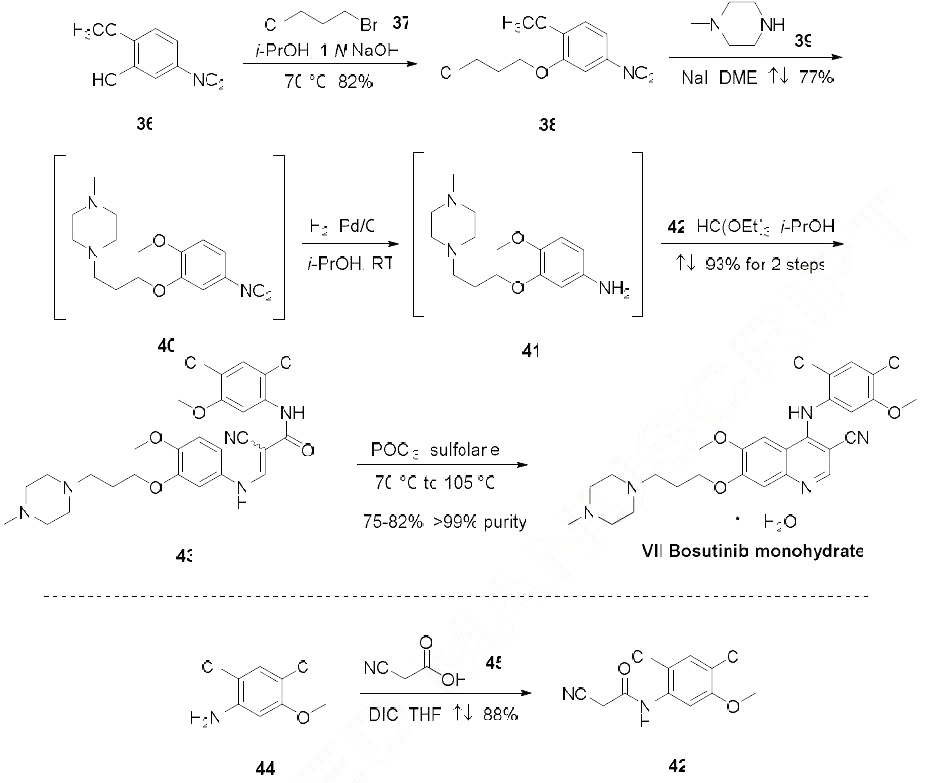
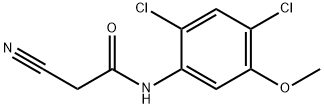
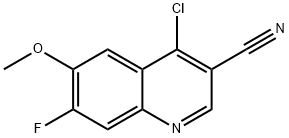
Several synthetic routes to bosutinib have been reported, including synthetic work for scale up and processing to obtain pure salt forms of bosutinib for pharmaceutical applications.56-59 The current manufacturing route begins with reaction of 2-methoxy-5-nitrophenol (36) and 1-bromo-3- chloropropane (37) to provide aryl chloroether 38 in 82% yield. Reaction of 38 with Nmethylpiperazine (39) and NaI in refluxing DME provided the functionalized aryl-nitro-piperazine 40 (77% yield), which was converted directly to aniline 41 under hydrogenolysis conditions. Aniline 41 was then reacted with triethyl orthoformate and aryl cyanoamide 42, which was generated in one step from 2,4-dichloro-5-methoxy-aniline (44), 1,3-diisopropylcabodiimide (DIC), and cyanoacetic acid (45) under refluxing conditions, to yield advanced intermediate 43 (93% over 2 steps). Finally, conversion of 43 to bosutinib was facilitated by a POCl3-promoted cyclization in the presence of sulfolane. As shown in Scheme 8, employment of carefully optimized conditions for the isolation of bosutinib hydrate (VII) provided material in 75-82% yields and >99% purity.
Properties of Bosutinib Monohydrate
| form | Solid |
| color | White to off-white |
Safety information for Bosutinib Monohydrate
Computed Descriptors for Bosutinib Monohydrate
Bosutinib Monohydrate manufacturer
Alembic Pharmaceuticals Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

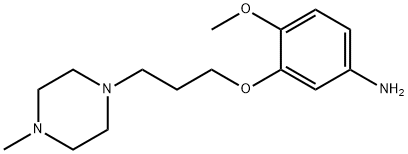
![1-[3-(2-Methoxy-5-nitrophenoxy)propyl]-4-Methylpiperazine](https://img.chemicalbook.in/CAS/20150408/GIF/846023-54-9.gif)

![4-[(2,4-Dichloro-5-methoxyphenyl)amino]-7-fluoro-6-methoxy-3-quinolinecarbonitrile](https://img.chemicalbook.in/CAS2/GIF/622369-46-4.gif)
You may like
-
 918639-08-4 Bosutinib monohydrate 98%View Details
918639-08-4 Bosutinib monohydrate 98%View Details
918639-08-4 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1