BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE
Synonym(s):Phosphonic acid bis(2,2,2-trifluoroethyl) ester
- CAS NO.:92466-70-1
- Empirical Formula: C4H5F6O3P
- Molecular Weight: 246.04
- MDL number: MFCD00063314
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 17:06:36
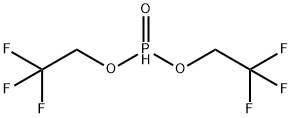
What is BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE?
The Uses of BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE
Bis(2,2,2-trifluoroethyl) phosphite was used in the synthesis of bis(2,2,2-trifluoroethyl phosphorochloridate. It was also employed as reagent for the synthesis of mono- and diesters of phosphorous acid.
Synthesis Reference(s)
Synthesis, p. 410, 1984 DOI: 10.1055/s-1984-30856
Synthesis
The synthesis method of Bis(2,2,2-trifluoroethyl) Phosphite is as follows: A solution of phosphorus trichloride (43.5 mL, 0.50 mol) in dichloromethane (100 mL) was placed in an oven dried 1L round bottom flask. A reflux condenser and pressure equalizing addition funnel were attached to the round bottom flask. The addition funnel was charged with a solution of 2-methyl-2-propanol (48.0 mL, 0.50 mol) in dichloromethane (100 mL), and then the contents of the addition funnel were added in a dropwise manner to the round bottom flask under argon gas at 0 ℃over the course of 30 min. The reaction mixture was allowed to stir for an additional 30 min, then the addition funnel is recharged with a solution of 2,2,2-trifluoroethanol (70.0 mL, 0.96 mol) and dichloromethane (100 mL) and then the contents of the addition funnel were added in a dropwise manner to the round bottom flask under argon gas at 0 ℃ over the course of 30 min. The reaction mixture was then allowed to stir at RT overnight. The gaseous vapors were then degassed into NaOH (30.2 g, 0.98 mol) in 200 mL of water over the course of 8 hrs. The solvents were removed by rotary evaporation and the crude material was fractionally distilled under reduced pressure yielding Bis(2,2,2-trifluoroethyl) Phosphite (100.89 g, 0.41 mol, 82.2%) as colorless liquid.
Properties of BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE
| Boiling point: | 43-44 °C/2 mmHg (lit.) |
| Density | 1.545 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 169 °F |
| form | Liquid |
| color | Colorless to Almost colorless |
Safety information for BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BIS(2,2,2-TRIFLUOROETHYL) PHOSPHITE
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran

![[2-(2-AMINO-6-CHLORO-PURIN-9-YL)-ETHOXYMETHYL]-PHOSPHONIC ACID BIS-(2,2,2-TRIFLUORO-ETHYL) ESTER](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB2208806.gif)
![ETHYL 2-[BIS(2,2,2-TRIFLUOROETHYL)PHOSPHONO] PROPIONATE](https://img.chemicalbook.in/CAS/GIF/107905-52-2.gif)
![BIS-(2,2,2-TRIFLUOROETHYL) METHOXYCARBONYLMETHYL PHOSPHONATE, [1,2-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure6/GIF/CB8231707.gif)
![ETHYL [BIS(2,2,2-TRIFLUOROETHOXY)PHOSPHINYL]ACETATE](https://img.chemicalbook.in/CAS/GIF/124755-24-4.gif)
![[BIS-(2,2,2-TRIFLUORO-ETHOXY)-PHOSPHORYL]-BROMO-ACETIC ACID METHYL ESTER](https://img.chemicalbook.in/StructureFile/ChemBookStructure6/GIF/CB3421505.gif)
You may like
-
 Bis(2,2,2-trifluoroethyl) Phosphite CAS 92466-70-1View Details
Bis(2,2,2-trifluoroethyl) Phosphite CAS 92466-70-1View Details
92466-70-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9