beta-Sitosterol
Synonym(s):β-Sitosterol;beta-Sitosterol;22,23-Dihydrostigmasterol;24α-Ethylcholesterol;5-Stigmasten-3β-ol
- CAS NO.:83-46-5
- Empirical Formula: C29H50O
- Molecular Weight: 414.72
- MDL number: MFCD00003631
- EINECS: 201-480-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
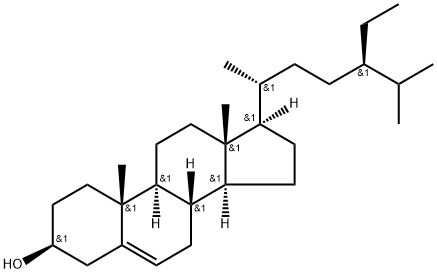
What is beta-Sitosterol?
Description
24α-ethyl Cholesterol is a naturally occurring plant sterol with antioxidant, anticancer, anti-
Chemical properties
White powder
The Uses of beta-Sitosterol
A common sterol in plants. Isolated from wheat germ oil, corn oil. Antilipemic. Used in the treatment of prostatic adenoma.
The Uses of beta-Sitosterol
antiinflammatory, immunomodulator
The Uses of beta-Sitosterol
A common sterol in plants. Isolated from wheat germ oil, corn oil. Antilipemic. Used in the treatment of prostatic adenoma.This compound is a contaminant of emerging concern (CECs)
What are the applications of Application
β-Sitosterol is a plant steroid which induces G2/M arrest
Definition
ChEBI: A phytosterols that is stigmast-5-ene substituted by a beta-hydroxy group at position 3.
Definition
A steroid with long aliphatic side chains (8–10 carbons) and at least one hydroxyl group. They are lipidsoluble and often occur in membranes. Examples are cholesterol and ergosterol.
Definition
sterol: Any of a group of steroidbasedalcohols having a hydrocarbonside-chain of 8–10 carbon atoms.Sterols exist either as free sterols oras esters of fatty acids. Animal sterols(zoosterols) include cholesterol andlanosterol. The major plant sterol(phytosterol) is beta-sitosterol, whilefungal sterols (mycosterols) includeergosterol.
General Description
Sitosterol, also called as β-sitosterol, is synthesized through the mevalonate and deoxy xylulose pathways. Synthetically, it is synthesized from stigmasterol by Δ22–23 alkene group hydrogenation. Structurally sitosterol differ from cholesterol with ethyl (sitosterol) group in C24 position of side chain(65). Sitosterol is a lipid component of membranes. It corresponds to a molecular mass of 414.71 g/mol).
Biochem/physiol Actions
Sitosterol promotes the antioxidant levels and is used as an effective anti-inflammatory, antiapoptotic and anticancer agent in medicinal preparations. It acts as a neutralizing agent for the venom from viper and cobra. Sitosterol acts on protein kinase C (PKC) and in the sphingomyelin cycle to mediate tumor progression inhibition.
Purification Methods
Crystallise -sitosterol from EtOH, MeOH, Me2CO, Me2CO/EtOH or Me2CO/MeOH. It has also been purified by zone melting. The acetate crystallises from MeOH or EtOH as plates with m 127128o and [] D 20 -41o (c 2, CHCl3). The benzoate crystallises from EtOH as needles with m 146-147o and [] D 20 –13.8o (c 2, CHCl3). [Fujimoto & Jacobson J Org Chem 29 3377, 3381 1964, Shoppee J Chem Soc 10431948, Heilbron et al. J Chem Soc 344, 347 1941, Beilstein 6 III 2696.]
Properties of beta-Sitosterol
| Melting point: | 136-140 °C(lit.) |
| Boiling point: | 473.52°C (rough estimate) |
| alpha | -28 º (c=2, CHCl3) |
| Density | 0.9540 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform: 20 mg/mL, clear, colorless |
| form | White solid |
| pka | 15.03±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | INSOLUBLE |
| Merck | 14,8556 |
| BRN | 1916165 |
| CAS DataBase Reference | 83-46-5(CAS DataBase Reference) |
| NIST Chemistry Reference | «beta»-Sitosterol(83-46-5) |
| EPA Substance Registry System | .beta.-Sitosterol (83-46-5) |
Safety information for beta-Sitosterol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for beta-Sitosterol
| InChIKey | KZJWDPNRJALLNS-VJSFXXLFSA-N |
beta-Sitosterol manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

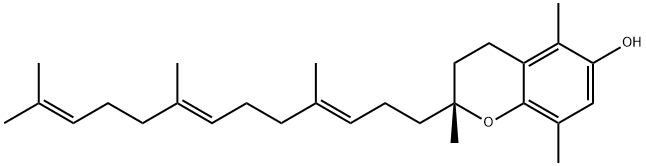
![[2R[2R*(4R*,8R*)]]-3,4-dihydro-2,5,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzopyran-6-ol](https://img.chemicalbook.in/CAS/GIF/16698-35-4.gif)

You may like
-
 β-Sitosterol (contains Campesterol) CAS 83-46-5View Details
β-Sitosterol (contains Campesterol) CAS 83-46-5View Details
83-46-5 -
 beta-Sitosterol >80% (GC) CAS 83-46-5View Details
beta-Sitosterol >80% (GC) CAS 83-46-5View Details
83-46-5 -
 b-Sitosterol ex. Rice Bran CAS 83-46-5View Details
b-Sitosterol ex. Rice Bran CAS 83-46-5View Details
83-46-5 -
 β-Sitosterol CAS 83-46-5View Details
β-Sitosterol CAS 83-46-5View Details
83-46-5 -
 Beta-Sitosterol (83-46-5) (C29h50o), 95%View Details
Beta-Sitosterol (83-46-5) (C29h50o), 95%View Details
83-46-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
