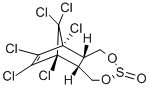
BETA-ENDOSULFAN
- CAS NO.:33213-65-9
- Empirical Formula: C9H6Cl6O3S
- Molecular Weight: 406.93
- MDL number: MFCD00151176
- EINECS: 625-635-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
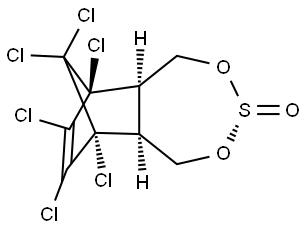
What is BETA-ENDOSULFAN?
Chemical properties
Endosulfan is a chlorinated cyclodiene insecticide. The pure product is a colorless crystalline solid. The technical product is a light to dark brown waxy solid. It has a rotten egg or sulfur odor.
Physical properties
Colorless to brown, nonflammable solid or crystals with a mild odor similar to terpene or sulfur dioxide.
The Uses of BETA-ENDOSULFAN
Insecticide for vegetable crops.
The Uses of BETA-ENDOSULFAN
β-Endosulfan may be used as an analytical pesticide reference standard for the determination of the analyte in water samples, virgin olive oil, aged contaminated Ethiopian soils and human fluids by various chromatography techniques.
General Description
Brown crystals. Melting point 208-210°C. Used as an insecticide.
Air & Water Reactions
Insoluble in water. Reacts slowly with water to generate sulfur dioxide.
Reactivity Profile
BETA-ENDOSULFAN is a sulfite ester of a chlorinated cyclic diol. Decomposed rapidly by alkali to generate sulfur dioxide. Decomposed by acid. Incompatible with strong oxidizing and reducing agents. may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Health Hazard
ACUTE/CHRONIC HAZARDS: Highly toxic by ingestion, inhalation, and skin absorption.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Environmental Fate
Soil. Metabolites of endosulfan identified in soils included endosulfandiol, endosulfanhydroxy
ether, endosulfan lactone and endosulfan sulfate (Martens, 1977; Dreher and
Podratzki, 1988). These compounds, including endosulfan ether, were also reported as
metabolites identified in aquatic systems (Day, 1991). In aerobic soils, b-endosulfan is
converted to the corresponding alcohol and ether (Perscheid et al., 1973). Endosulfan
sulfate was the major biodegradation product in soils under aerobic, anaerobic and flooded
conditions (Martens, 1977). In flooded soils, endolactone was detected only once whereas
endodiol and endohydroxy ether were identified in all soils under these conditions. Under
anaerobic conditions, endodiol formed in low amounts in two soils (Martens, 1977).
Indigenous microorganisms obtained from a sandy loam degraded b-endosulfan to
endosulfan diol. This diol was converted to endosulfan a-hydroxy ether and trace amounts
of endosulfan ether and both were degraded to endosulfan lactone (Miles and Moy, 1979).
Plant. In addition, endosulfan sulfate was formed when endosulfan was translocated
from the leaves to roots in both bean and sugar beet plants (Beard and Ware, 1969). In
tobacco leaves, b-endosulfan hydrolyzed into endosulfandiol (Chopra and Mahfouz, 1977).
Stewart and Cairns (1974) reported the metabolite endosulfan sulfate was identified in
potato peels and pulp at concentrations of 0.3 and 0.03 ppm, respectively. They also
reported that the half-life for the oxidative conversion of b-endosulfan to endosulfan sulfate
was 800 days.
In carnation plants, the half-lives of b-endosulfan stored under four different conditions,
non-washed and exposed to open air, washed and exposed to open air, non-washed
and placed in an enclosed container and under greenhouse conditions were 23.40, 12.64,
37.42 and 7.62 days, respectively (Ceron et al., 1995).
Surface Water. Endosulfan sulfate was also identified as a metabolite in a survey of
11 agricultural watersheds located in southern Ontario, Canada (Frank et al., 1982). When
endosulfan (a- and b- isomers, 10 mg/L) was added to Little Miami River water, sealed
and exposed to sunlight and UV light for 1 week, a degradation yield of 70% was observed.
After two and four weeks, 95% and 100% of the applied amount degraded. The major
degradation product was identified as endosulfan alcohol by IR spectrometry (Eichelberger
and Lichtenberg, 1971).
Photolytic. Thin films of endosulfan on glass and irradiated by UV light (l >300 nm)
produced endosulfan diol with minor amounts of endosulfan ether, lactone, a-hydroxyether
and other unidentified compounds (Archer et al., 1972). Gaseous b-endosulfan subjected
to UV light (l >300 nm) produced endosulfan ether, endosulfan diol, endosulfan sulfate,
endosulfan lactone, a-endosulfan and a dechlorinated ether (Schumacher et al., 1974).
Irradiation of b-endosulfan in n-hexane by UV light produced the photoisomer a-endosulfan
(Putnam et al., 1975). When an aqueous solution containing endosulfan was photooxidized
by UV light at 90–95°C, 25, 50 and 75% degraded to carbon dioxide after 5.0, 9.5
and 31.0 hours, respectively (Knoevenagel and Himmelreich, 1976).
Chemical/Physical. Endosulfan detected in Little Miami River, OH was readily hydrolyzed
to a compound tentatively identified as endosulfan diol (Eichelberger and Lichtenberg,
1971). Sulfuric acid is also an end product of hydrolysis (Kollig, 1993). The hydrolysis
half-lives at pH values (temperature) of 3.32 (87.0°C), 6.89 (68.0°C) and 8.69 (38.0°C)
were calculated to be 2.7, 0.07 and 0.04 days, respectively (Ellington et al., 1988). Greve
and Wit (1971) reported the hydrolysis half-lives of b-endosulfan at 20°C and pH values
of 7 and 5.5 were 37 and 187 days, respectively.
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Waste Disposal
A recommended method for disposal is burial 18 in deep in noncropland, away from water supplies, but bags can be burned. Large quantities should be incinerated at high temperature in a unit with effluent gas scrubbing. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of BETA-ENDOSULFAN
| Melting point: | 209°C |
| Boiling point: | 449.7±45.0 °C(Predicted) |
| Density | 1.6568 (estimate) |
| vapor pressure | 450 at 20 °C (shake flask-GLC, Bowman and Sans, 1983) |
| Flash point: | 11 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform: Slightly Soluble; Methanol: Slightly Soluble |
| form | neat |
| Water Solubility | 0.28mg/L(25 ºC) |
| BRN | 2950317 |
| Henry's Law Constant | 0.022 at 5 °C, 0.037 at 15 °C, 0.043 at 20 °C, 0.065 at 25 °C, 0.80 at 35 °C:in 3% NaCl solution:
0.20 at 5 °C, 0.37 at 15 °C, 0.46 at 25 °C, 0.56 at 35 °C (gas stripping-GC, Cetin et al., 2006) |
| Exposure limits | ACGIH TLV: TWA 0.1 mg/m3. |
| Stability: | Light Sensitive |
| NIST Chemistry Reference | Endosulfan ii(33213-65-9) |
| EPA Substance Registry System | .beta.-Endosulfan (33213-65-9) |
Safety information for BETA-ENDOSULFAN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for BETA-ENDOSULFAN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8