Bilastine
- CAS NO.:202189-78-4
- Empirical Formula: C28H37N3O3
- Molecular Weight: 463.62
- MDL number: MFCD09837814
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
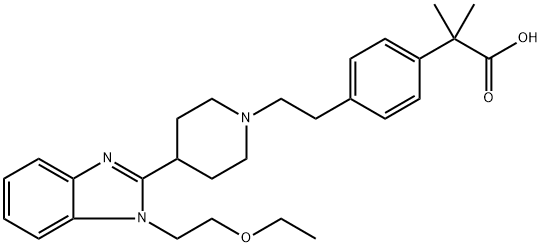
What is Bilastine?
Description
Bilastine, a potent and selective histamine H1 receptor antagonist, was
approved in Europe in 2010 for the treatment of allergic rhinoconjunctivitis
(AR) and urticaria (hives or skin rash). The original synthesis of bilastine involves alkylation of 2-piperidinyl-1H-benzimidazole with a phenethyltosylate,
the para position of which is substituted with a dimethyloxazoline
moiety serving as a masked carboxylic acid group. Alkylation of the
benzimidazole nitrogen with 2-chloroethyl ethyl ether followed by
unmasking of the oxazoline moiety with sulfuric acid provided bilastine.
In two major clinical trials, bilastine was effective
at relieving allergic rhinitis as assessed by measuring the severity of nasal (obstruction, rhinorrhea, itching, sneezing) and nonnasal (ocular
itching, tearing, ocular redness, itching of ears, and/or palate) symptoms.
Description
Bilastine is a histamine H1 receptor antagonist (IC50 = 180 nM). It is selective for the histamine H1 receptor in a panel of 30 receptors in vitro at 100 μM. Bilastine prevents microvascular extravasation (ED50 = 185 μg/kg, i.v.), bronchospasm (ED50 = 4.6 μg/kg, i.v.), and systemic anaphylaxis (ED50 = 0.2 μg/kg, p.o.) induced by subcutaneous histamine in guinea pigs. It prevents anaphylaxis induced by subcutaneous administration of ovalbumin or dinitrophenylated human albumin (DNP) in sensitized rats when administered at doses of 7.6 and 6.0 mg/kg, respectively. Formulations containing bilastine have been used in the treatment of urticaria and allergic rhinitis.
Originator
FAES FARMA, S.A. (Spain)
The Uses of Bilastine
Bilastine is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
The Uses of Bilastine
Labelled Bilastine. It is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Background
Bilastine is a novel new-generation antihistamine that is highly selective for the H1 histamine receptor, has a rapid onset and prolonged duration of action.
Indications
For symptomatic relief of nasal and non-nasal symptoms of seasonal rhinitis in patients 12 years of age and older and for symptomatic relief in chronic spontaneous urticaria in patients 18 years of age and older .
Definition
ChEBI: Bilastine is a member of benzimidazoles.
brand name
Bilaxten
Pharmacokinetics
Bilastine is an antiallergenic and acts to reduce allergic symptoms such as nasal congestion and urticaria .
Clinical Use
Bilastine is a selective histamine H1 antagonist approved for the treatment of allergic rhinoconjunctivitis and urticaria (hives). This drug, which has proven to be well tolerated in toxicology profiling, 46 was discovered by the Spainsh firm FAES Farma and was approved by the European Union in 2010.
Synthesis
In 2011, Collier and
co-workers published a communication describing both the original
synthesis of bilastine and an improved route which was
amenable to gram-scale production. Collier?ˉs second generation
route, shown below, relies upon a convergent approach
involving the union of piperidinyl benzimidazole 49 with fully
functionalized phenethyl electrophile 48.Coupling the commercially
available bromophenyl acetate 44 with cyclic trioxatriborinane
45 under conventional Suzuki conditions furnished styrene
46 in good yield. Alternatively, this vinylation reaction was also
performed under Stille conditions with tributyl vinyl stannane in
83% yield. Hydroboration¨Coxidation of 46 delivered phenethyl
alcohol 47 which was then immediately mesylated under basic
conditions in toluene to produce adduct 48. This sulfonate was
then reacted with piperidine 49 (whose preparation is described
in Scheme 7) followed by saponification of the resulting ester 50
to arrive at bilastene (VI) in 26% overall yield from 44.
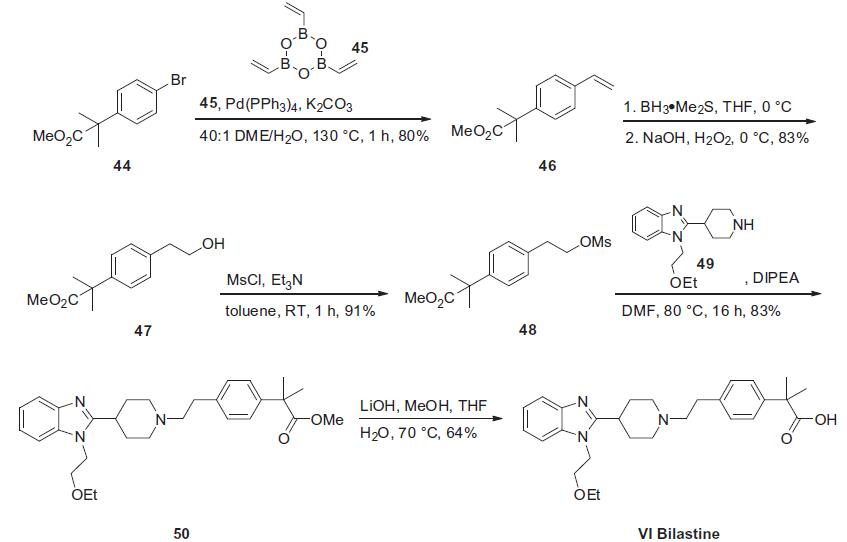
For the preparation of bilastine piperidine 49,
commercially available piperidine 51 was first protected as the Boc-carbamate 52 prior to alkylation of the benzimidazole nitrogen
atom with 1-chloro-2-ethoxyethane 53, providing compound
54. The Boc group of 54 was removed under acidic conditions to
give fragment 49. This sequence produced the desired piperidine
component in 86% overall yield from 51.
Drug interactions
Potentially hazardous interactions with other drugs
Antivirals: concentration possibly increased by
ritonavir.
Grapefruit juice: concentration of bilastine reduced.
Absorption
Bilastine has a Tmax of 1.13 h . The absolute bioavailability is 61%. No accumulation observed with daily dosing of 20-100 mg after 14 days. Cmax decreased by 25 % and 33% when taken with a low fat and high fat meal compared to fasted state. Administration with grapefruit juice decreased Cmax by 30%.
Metabolism
Bilastine does not interact with the cytochrome P450 system and does not undergo significant metabolism in humans .
Metabolism
Not significantly metabolised. Almost 95% of the administered dose was recovered in urine (28.3%) and faeces (66.5%) as unchanged bilastine
Toxicity
The most common adverse effects experienced during clinical trials were abdominal pain, dizziness, headache, and somnolence . Bilastine is associated with Q/T prolongation. The no observed adverse effect level of bilastine is 1200 mg/kg/day in rats and 125 mg/kg/day in dogs .
Properties of Bilastine
| Melting point: | 202 °C |
| Boiling point: | 639.1±55.0 °C(Predicted) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | DMSO:49.3(Max Conc. mg/mL);106.34(Max Conc. mM) |
| form | powder to crystal |
| pka | 4.40±0.10(Predicted) |
| color | White to Light yellow |
Safety information for Bilastine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bilastine
Abamectin manufacturer
SHOBHA LIFE SCIENCES PRIVATE LIMITED
Meenaakshi Molecules Pvt Ltd
Aalidhra Pharmachem Pvt Ltd
Integrin Life Sciences. Pvt. Ltd.
Centaur Pharmaceuticals Pvt Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![4-[1-(4,5-DIHYDRO-4,4-DIMETHYL-2-OXAZOLYL)-1-METHYLETHYL]-BENZENEETHANOL](https://img.chemicalbook.in/CAS/GIF/361382-26-5.gif)
![2-(1-(4-(2-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)propan- 2-yl)phenethyl)piperidin-4-yl)-1H-benzo[d]imidazole](https://img.chemicalbook.in/CAS/20180527/GIF/202189-81-9.gif)
![2-Propenoic acid, 3-[3-[(phenylaMino)sulfonyl]phenyl]-, ethyl ester, (2E)-](https://img.chemicalbook.in/CAS/GIF/1137621-29-4.gif)

![2-Propenoic acid, 3-[3-[(phenylaMino)sulfonyl]phenyl]-, Methyl ester, (2E)-](https://img.chemicalbook.in/CAS/GIF/866323-86-6.gif)
![2-(2-(4-(2-(4-(1-(2-ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)propan-2-yl)-4,4-dimethyl-4,5-dihydrooxazole](https://img.chemicalbook.in/CAS/20180703/GIF/202189-77-3.gif)
![Benzeneethanol, 4-[1-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-1-methylethyl]-, 4-methylbenzenesulfonate (ester)](https://img.chemicalbook.in/CAS/20180703/GIF/202189-76-2.gif)
You may like
-
 Bilastine 98%View Details
Bilastine 98%View Details -
 Bilastine 98%View Details
Bilastine 98%View Details -
 Bilastine 98%View Details
Bilastine 98%View Details -
 Bilastine 99%View Details
Bilastine 99%View Details -
 Bilastine 98%View Details
Bilastine 98%View Details -
 BILASTINE 98%View Details
BILASTINE 98%View Details -
 Bilastine 95-99 %View Details
Bilastine 95-99 %View Details
202189-78-4 -
 Bilastine 98%View Details
Bilastine 98%View Details