barium hydroxyapatite
- CAS NO.:12356-34-2
- Empirical Formula: Ba5O13P3-
- Molecular Weight: 987.548483
- SAFETY DATA SHEET (SDS)
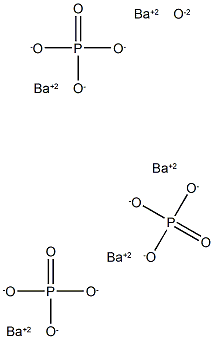
What is barium hydroxyapatite?
Description
Barium Hydroxyapatite is prepared by the aqueous reaction of
a soluble barium salt with trisodium phosphate:
10BaCl2 (aq) + 6Na3PO4 (aq)
?Ba10(OH)2(PO4)6 (s) + 18NaCl (aq) + 2HCl
Alternately, it can be prepared by the aqueous reaction
of the monobasic salt and the hydroxide at about
60–80°C with addition of NaOH to control pH to
a minimum of 10–11.
3Ba(H2PO4)2 (aq) + 2Ba(OH)2 (solid)
?Ba5OH(PO4)3 (solid) + 8H2O
Its molecular weight is 1728.3662 g/mol and the CAS
number of 12356-34-2. Barium readily forms mixed
hydroxyapatties with both calcium and strontium
hydroxyapatites. These hydroxyapatites can be OH deficient,
with the charge balance maintained by Ba2+, Sr2+
or Ca2+ vacancies. It has been found that Ba2+ ions are
more difficult to be incorporated into hydroxyapatite
crystals compared to Sr22+ions. In a mixture with
increasing Ba2+content, the particles grew and finally
turned into pure rod-shaped barium hydroxyapatite
particles with a size of ca. 0.2μ.
Preparation
When barium hydroxyapatite, (Ba10(PO4)6(OH)2,
(abbreviated-BaHAp) was synthesized by a wet method
using Ba(OH)2·8H2O and (NH4)2HPO4 as starting materials,
the Ba-HAp obtained had a Ba/P atomic ratio of
1.76 and contained CO3 groups. Thus, any solution
method to prepare this compound must be protected
fromthe ambient atmosphere in order to obtain a pure salt.
Carbonated barium hydroxyapatite Ba10(PO4)6
(OH)2-2x(CO3)x, where X= 0.30–0.57, produced BaHAP
particles with different Ba/P molar ratios. These were
prepared by a wet method and CO32- ions were incorporated
into OH- sites of a BaHAP lattice during the preparation
at high pH solution. The obtained BaHAP particles
were well crystallized and showed a high thermostability.
On elevating the mixing temperature of the H3PO4 and
Ba(OH)2 solutions, the mean particle size of BaHAP particles
decreased and their specific surface area increased.
The amount of CO2 adsorbed irreversibly on BaHAP
particles increased as their Ba/P molar ratio increased.
In another study, carbonate-containing barium
hydroxyapatite was prepared by solid-state reactions in air at 950°C. Obtained samples were investigated by
X-ray powder diffraction and IR spectroscopy.
Safety information for barium hydroxyapatite
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran








You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1