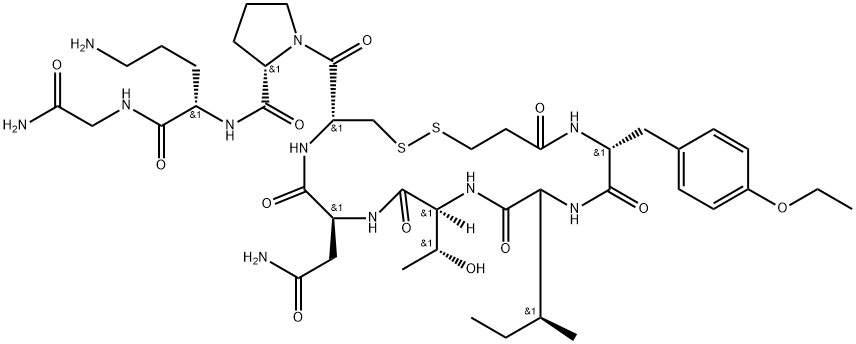
Atosiban
Synonym(s):1-(3-Mercaptopropanoic acid)-2-(O-ethyl-D-tyrosine)-4-L-threonine-8-L-ornithineoxytocin;1-Deamino-2-D-Tyr-(O-ethyl)-4-Thr-8-ornoxytocin;Tractocile
- CAS NO.:90779-69-4
- Empirical Formula: C43H67N11O12S2
- Molecular Weight: 994.19
- MDL number: MFCD00672436
- EINECS: 806-815-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Atosiban?
Absorption
In women receiving 300 μg/min by infusion for 6-12 h, average steady state concentrations of 442 ng/mL were reached within 1 h . Steady state concentrations increase proportionally to dosage.
Toxicity
No systemic toxicities were found in rat and dog studies at dosages equivalent to 10 times normal human exposure . It is thought that the risk of toxicity is low due to the short duration of action and short half life of atosiban .
Description
Atosiban was introduced in the UK as an injectable inhibitor of preterm labor, a major cause of infant morbidity and mortality. This peptidic oxytocin analog is an antagonist of the vasopressin V1a receptor and of the oxytocin receptor which is found in dramatically increased concentration in the uterine myometrium of pregnant women near term. It competitively inhibited contractions in the pregnant guinea pig uterus induced by oxytocin and vasopressin. In a multicenter, double-blind, placebo-controlled trial, treatment with atosiban caused pregnancy prolongation for up to 7 days in women with more than 28 weeks of gestation. In a comparative clinical trial, atosiban showed a comparable tocolytic action (uterine relaxant) to ritodrine but the former was significantly better tolerated, especially with regards to maternal cardiovascular side effects. In healthy volunteers, plasma levels of atosiban decreased bi-exponentially with an initial and a terminal half-life of 21 min and 1.7h respectively.
Originator
Ferring AB (Sweden)
The Uses of Atosiban
Atosiban is an oxytocin receptor blocking agent in the treatment of experimental endometriosis and was shown exhibit significant therapeutic efficiency.
The Uses of Atosiban
Premature labor
The Uses of Atosiban
Atosiban has been used:
- as an oxytocin receptor antagonist
- in the calcium mobilization assay for Z factor determination in uterine myometrium (UT-myo cells) and as a therapeutic agent to inhibit preterm labor
- to inhibit the activation of oxytocin-receptor-expressing neurons in the parabrachial nucleus of mice (OxtrPBN)
Background
Atosiban is an inhibitor of the hormones oxytocin and vasopressin. It is used intravenously to halt premature labor. Although initial studies suggested it could be used as a nasal spray and hence would not require hospital admission, it is not used in that form. Atobisan was developed by the Swedish company Ferring Pharmaceuticals. It was first reported in the literature in 1985. Atosiban is licensed in proprietary and generic forms for the delay of imminent pre-term birth in pregnant adult women.
Indications
Atosiban is indicated for use in delaying imminent pre-term birth in pregnant adult women with:
What are the applications of Application
Atosiban is a peptide oxytocin receptor inhibitor
Indications
Atosiban is an analogue of oxytocin that is modified at positions 1, 2, 4, and 8. It is a competitive inhibitor of oxytocin binding. Early studies have demonstrated that this drug does decrease and stop uterine contractions. Atosiban is not available for use in the United States.
Definition
ChEBI: Atosiban is an oligopeptide.
Manufacturing Process
BocGly resin (3.0 g, 3 meq) was placed in the reaction vessel of a Vega Model
50 semiautomatic peptide synthesizer. The peptide was built up by increments
on the resin in accordance with Tables 1 and 2.
Activation of the amino acid was carried out by dissolving 10 meq of a
suitably protected amino acid, 15 meq of hydroxy benzotriazole and 10 meq
of dicyclohexylcarbodiimide in DMF (70 ml), whereupon the mixture was left
at room temperature for 1 h (asparagine and glutamine were activated at 0°C
for 15 min), whereupon the precipitate was filtered off, and the filtrate was
treated the activated amino acid in Table 1 (step 7). The completion of the
coupling step was checked by the method of Kaiser (Anal. Biochem. 34, 595
(1970)) after the cycle had been completed (step 9). If the test was positive
(coupling yield below 99%), the cycle was repeated starting from step 7. If
the test was negative, the termination procedure was performed according to
Table 2. When the whole sequence had been coupled, the resin was placed on
a filter and washed repeatedly with methanol. The dried product was placed in
a glass vessel and cooled in an ethanol-dry ice bath and suspended in
methanol (about 100 ml). The mixture was then saturated with sodium-dried
ammonia to achieve approximately 50% concentration. Then the vessel was
placed in a steel cylinder and left at room temperature for two days. After the
pressure had been relieved, the product was filtered, and the residue was
extracted with hot (about 100°C) DMF (2x100 ml). The filtrate and the extract
were combined and evaporated. The residue was dissolved in a small amount
of hot DMF, and methanol was added to the coupling point. The precipitate
was collected by filtration and washed on the filter with methanol. After drying
in vacuum, the purity was checked by thin-layer chromatography. Yield about
2.8 g.
100 mg of the above described protected peptide were placed in a 100 ml round-bottom flask, and dry nitrogen was flushed through for about 15 min.
50 ml of sodium-dried ammonia were distilled in, and the protective group
was removed from the product by adding sodium until blue color remained in
the solution for 15 sec. The excess of sodium was destroyed by adding of
ammonium chloride. Ammonia was removed in a nitrogen stream, and the
residue was dissolved in 1 liter of methanol. The pH of the solution was
adjusted to about 4 with concentrated acetic acid, and the solution was then
titrated with 0.1 mM of iodine in methanol to brownish color. The mixture was
stirred with 3 g of Dowex 50x2 ion exchanger in chloride form for 10 min at
room temperature. The ion exchanger was removed by filtration, and the
filtrate was evaporated to dryness. The residue was dissolved in 3 ml of 20%
acetic acid and purified by chromatography on Sephadex G-25 with 20%
acetic acid as eluent. The final purification was achieved by reverse phase
HPLC. The purity of the product was determined on a HPCL column μ-
Bondapak C-18 in 45% ethanol and 55% 5 mM trifluoroacetic acid in water.
The column was supplied by Water Associates, Inc., Millford, Mass., U.S.A. The
purity of the product was also shown by amino acid analysis.
brand name
Tractocile, Antocin
Therapeutic Function
Oxytocin antagonist
Biochem/physiol Actions
Atosiban efficiently prevent preterm uterine contractions without any major cardiovascular, pulmonary or central nervous system side effects. It has potential to treat preterm labour.
Pharmacokinetics
Atosiban reduces the frequency of uterine contractions to delay pre-term birth in adult females and induces uterine quiescence .
Anticancer Research
The antitumor effects of the essential oil of O. falcata were tested in transplantedmurine H22 solid tumors in vivo. Growth inhibition in H22 solid tumors was moderate(Yang et al. 2013).
Metabolism
There are two metabolites of atosiban created through the cleavage of the peptide bond between ornithine and proline which is thought to be facilitated by prior cleavage of the disulfide bridge . The larger fragment remains active as an antagonist of oxytocin receptors but is 10 times less potent than the parent molecule. At a dosage of 300 μg/min the ratio of parent molecule to the main metabolite was observed to be 1.4 at the second hour and 2.8 at the end of infusion .
storage
Store at -20°C
Properties of Atosiban
| Melting point: | >165oC (dec.) |
| Boiling point: | 1469.0±65.0 °C(Predicted) |
| Density | 1.254±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | H2O: ≤100 mg/mL |
| form | solid |
| pka | 12.81±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble to 50 mg/ml in water |
| CAS DataBase Reference | 90779-69-4(CAS DataBase Reference) |
Safety information for Atosiban
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Atosiban
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Atosiban 98.00% CAS 90779-69-4View Details
Atosiban 98.00% CAS 90779-69-4View Details
90779-69-4 -
 Atosiban CAS 90779-69-4View Details
Atosiban CAS 90779-69-4View Details
90779-69-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1