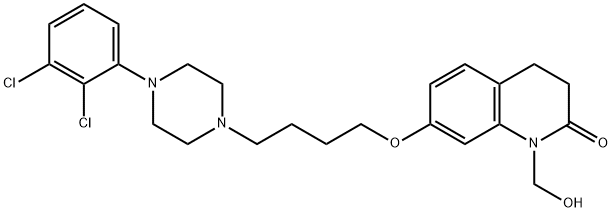
Aripiprazole Lauroxil
- CAS NO.:1259305-29-7
- Empirical Formula: C36H51Cl2N3O4
- Molecular Weight: 660.71
- MDL number: MFCD26967976
- EINECS: 826-270-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
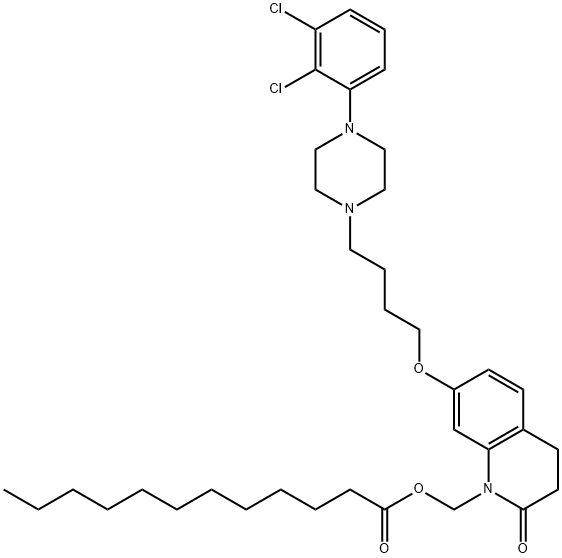
What is Aripiprazole Lauroxil?
Absorption
Following a single extended-release intramuscular injection of aripiprazole lauroxil, aripiprazole can be detected in the systemic circulation from 5 to 6 days and is continued to be released for an additional 36 days. The concentrations of aripiprazole increases with consecutive doses of aripiprazole lauroxil and the steady state is reached following the fourth monthly injection . The systemic exposure to aripiprazole was similar when comparing deltoid and gluteal intramuscular injections .
Toxicity
LD50 in rat following intramuscular injection was >60 mg aripiprazole equivalents . Oral LD50 of aripiprazole in female rat, male rat, and monkey were 705 mg/kg, 965 mg/kg, and >2000 mg/kg, respectively . Most common adverse reaction of aripiprazole was akathisia. A case of drug overdosage occurred followinga acute ingestion of 1260 mg aripiprazole, which is approximately 42 times the maximum recommended daily dose. Overdose was associated with vomiting, somnolence, and tremor . Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia .
Aripiprazole is an antipsychotic drug that may develop Neuroleptic Malignant Syndrome (NMS), which is manifested with hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability. In case of NMS, aripiprazole should be discontinued immediately, and intensive symptomatic treatment and medical monitoring should be initiated .
Description
Aripiprazole lauroxil is a long acting injectable (LAI) pro-drug formulation of aripiprazole approved in the U.S. for the treatment of schizophrenia. Aripiprazole lauroxil is a dopamine D2 receptor partial antagonist, a 5-HT2A antagonist, and a 5- HT1A partial agonist that was developed by Alkermes. It was Aripiprazole lauroxil is a long acting injectable (LAI) pro-drug formulation of aripiprazole approved in the U.S. for the treatment of schizophrenia.28 Aripiprazole lauroxil is a dopamine D2 receptor partial antagonist, a 5-HT2A antagonist, and a 5- HT1A partial agonist that was developed by Alkermes. It was
Indications
Aripiprazole lauroxil is indicated for the treatment of schizophrenia and related psychotic disorders.
Background
Aripiprazole lauroxil is a long-acting injectable atypical antipsychotic drug used in the treatment of schizophrenia in adult patients. It is a prodrug of aripiprazole, which acts as a partial agonist at the D2 and 5-HT1A receptors, and as an antagonist at the 5-HT2A receptors .
Affecting about 1% of the adult population in the United States and approximately 26 million people worldwide, schizophrenia is a chronic neurological disorder that may result in impairments in cognition and executive functions . The quality of life in patients is greatly reduced due to negative health outcomes, and oftentimes the patients are faced with social stigma and discriminations. Schizophrenia is characterized by positive symptoms such as delusions, hallucinations, thought disorders, and catanoia, and negative symptoms that include social withdrawal, anhedonia, and flattening of emotional responses . D2 receptors have been the most common target for antipsychotic agents used in the treatment of schizophrenia: the positive symptoms are thought to arise from overactivity in the mesolimbic dopaminergic pathway activating D2 receptors, whereas negative symptoms may result from a decreased activity in the mesocortical dopaminergic pathway with D1 receptors predominating . In a randomized, double-blind clinical trial, treatment of aripiprazole lauroxil in adult patients with schizophrenia resulted in improvement of positive and negative symptoms scores at day 85 of treatment .
Aripiprazole lauroxil was initially approved by the FDA in October 2015 under the market name Aristada for the treatment of schizophrenia. It is administered via intramuscular injection, and requires the establishment of tolerability prior to dosing in treatment-na?ve patients . On July 2nd, a different formulation of aripiprazole lauroxil marketed as Aristada Initio was FDA-approved for immediate initiation of Aristada at any dose. The patients may receive Aristada Initio in combination with a single 30 mg oral dose of aripiprazole to achieve appropriate levels of aripiprazole more rapidly. Long-acting injectable aripiprazole lauroxil displayed comparable efficacy and safety to aripiprazole , and reduced dosing frequency improves patient adherence.
Definition
ChEBI: A dodecanoate ester obtained by formal condensation of the carboxy group of dodecanoic acid with the hydroxy group of 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-2-oxo-3,4-dihydroquinolin-1(2H)-yl]methanol. A prodrug for aripipraz le, it is used for treatment of schizophrenia.
Pharmacokinetics
Aripiprazole, which is a major pharmacological metabolite of aripiprazole lauroxil, serves to improve the positive and negative symptoms of schizophrenia by modulating dopaminergic signalling pathways. Aripiprazole lauroxil is reported to have minimal effects on sexual function or prolactin levels .
Synthesis
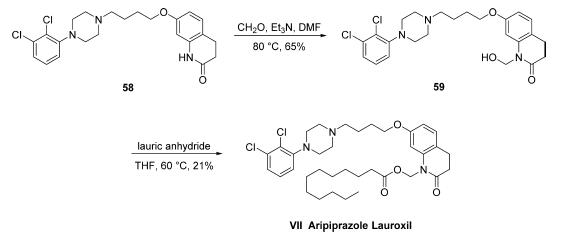
The synthesis of aripiprazole lauroxil has only been described on gram scale in the patent literature. Commercially available aripiprazole (58) was treated with formaldehyde to give hemiaminal 59 in 65% crude yield and was then heated with lauric anhydride to give aripiprazole lauroxil (VII) in 21% overall yield.
Metabolism
Aripiprazole lauroxil is hydrolyzed to form N-hydroxymethyl-aripiprazole via esterases. N-hydroxymethyl-aripiprazole undergoes a rapid, nonenzymatic spontaneous cleavage, or water-mediated hydrolysis, to form aripiprazole, which mainly contributes to the pharmacological actions of aripiprazole lauroxil. Aripiprazole is further metabolized by hepatic CYP3A4 and CYP2D6 to form dehydro-aripiprazole, which retains some pharmacological activity. Dehydro-aripiprazole displays affinities for D2 receptors similar to aripiprazole and represents 30-40% of the aripiprazole exposure in plasma . Cytochrome P450 2D6 is subject to genetic polymorphism, which results in pharmacokinetic differences among CYP2D6 metabolizer phenotypes and dosage adjustments accordingly .
Properties of Aripiprazole Lauroxil
| Melting point: | 83 - 84°C |
| Boiling point: | 781.7±60.0 °C(Predicted) |
| Density | 1.153±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 7.67±0.10(Predicted) |
| color | White to Off-White |
Safety information for Aripiprazole Lauroxil
Computed Descriptors for Aripiprazole Lauroxil
Abamectin manufacturer
Honour Lab Limited
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 ARIPIPRAZOLE LAUROXIL 98%View Details
ARIPIPRAZOLE LAUROXIL 98%View Details -
 1259305-29-7 98%View Details
1259305-29-7 98%View Details
1259305-29-7 -
 ARIPIPRAZOLE LAUROXIL 98%View Details
ARIPIPRAZOLE LAUROXIL 98%View Details
1259305-29-7 -
 Aripiprazole lauroxil 95% CAS 1259305-29-7View Details
Aripiprazole lauroxil 95% CAS 1259305-29-7View Details
1259305-29-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4