apovincamine
Synonym(s):Apovincamine;Methyl (13aS,13bS)-13a-ethyl-2,3,5,6,13a,13b-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate;Vinpocetine EP Impurity B
- CAS NO.:4880-92-6
- Empirical Formula: C21H24N2O2
- Molecular Weight: 336.43
- MDL number: MFCD00867558
- EINECS: 225-491-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-08 18:06:34
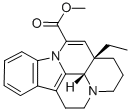
What is apovincamine?
Originator
Apovincamine,GC Promochem
The Uses of apovincamine
cis-Apovincamine (Vinpocetine USP Related Compound B), is a vinca alkaloid and a chemical precursor of Vinpocetine (V332500), a derivative of Vincamine with vasodilating activity. Vasodilator (cerebral).
Definition
ChEBI: Apovincamine is an alkaloid.
Manufacturing Process
Apovincamine is semisynthethetic derivative of (+)-vincamine. Vincamine is
alkaloid of Vinca minor. Alcoloid of Tabernaemontana rigida (Apocynaceae)
also is used as raw material for preparing of apovincamine. (+)- Vincamine
was transformed into oxime ester by reaction with NaNO2 in acetic acid at 0°C
-(+/-)-methyl-3-((12bR)-1-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-
a]quinolizin-1-yl)-2-(hydroxyimino)propanoate, which was resolved on the
isomers with dibenzoyl D-tartratic acid. (-)-Methyl 3-((12bR)-1-ethyl-
1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-1-yl)-2-
(hydroxyimino)propanoate was re-crystallized from methanol to afford (-)-
methyloxime ester, MP: 195°C.
2 g of above oxime methyl ester was heated in mixture of methanol (37.5 ml)
and conc. H2SO4 (13.5 ml) on water bath for 1 hour. The solution was poured
into ice-water (80 ml), basified with conc. NH4OH to pH 9, and extracted with
CH2Cl2 (3x20 ml). The combined extracts were dried (MgSO4), filtered,
evaporated in vacuum and the residue was re-crystallized from MeOH (5 ml)
to yield 1.32 g (72.5%) of (+)-apovincamine, MP: 160°-162°C.
Therapeutic Function
Vasodilator
Properties of apovincamine
| Melting point: | 160-162℃ |
| Boiling point: | 405.7±45.0 °C(Predicted) |
| Density | 1.30±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 7.82±0.60(Predicted) |
| form | neat |
| color | Off-White to Pale Grey |
Safety information for apovincamine
Computed Descriptors for apovincamine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![ethyl (41S,12R,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate](https://img.chemicalbook.in/CAS/20211123/GIF/77549-92-9.gif)
![ethyl (41R,13aS)-13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate](https://img.chemicalbook.in/CAS/20210111/GIF/77549-94-1.gif)
![methyl (41S,12S,13aS)-13a-ethyl-12-hydroxy-8-methoxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate](https://img.chemicalbook.in/CAS/20210111/GIF/57430-34-9.gif)
![methyl (41R,12S,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate](https://img.chemicalbook.in/CAS/20180808/GIF/18374-18-0.gif)
![(41S,12S,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylic acid](https://img.chemicalbook.in/CAS/20211123/GIF/59413-21-7.gif)
You may like
-
 Vinpocetine Related Compound B CAS 4880-92-6View Details
Vinpocetine Related Compound B CAS 4880-92-6View Details
4880-92-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
