anisidine
- CAS NO.:29191-52-4
- Empirical Formula: C7H9NO
- Molecular Weight: 123.15246
- EINECS: 249-496-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
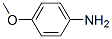
What is anisidine?
Chemical properties
Anisidine exists as ortho-, meta-, and paraisomers. They have characteristic amine (fishy) odors.
Definition
ChEBI: P-anisidine is a substituted aniline that is aniline in which the hydrogen para to the amino group has been replaced by a methoxy group. It is used as a reagent for the detection of oxidation products such as aldehydes and ketones in fats and oils. It has a role as a reagent and a genotoxin. It is a member of methoxybenzenes, a substituted aniline and a primary amino compound.
General Description
A fused crystalline solid or a reddish to yellowish oily liquid. More dense than water and insoluble in water. Very irritating to skin, eyes, and mucous membranes. Toxic by ingestion and skin contact. Used to make dyes.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Potential Exposure
Anisidines are used in the manufacture of azo dyes; pharmaceuticals; textile-processing chemicals Incompatibilities: Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine,bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some coatings and some forms of plastic and rubber.
Shipping
UN2431 Anisidines, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Waste Disposal
Dissolve in combustible solvent (alcohols, benzene, etc.) and spray solution into furnace equipped with afterburner and scrubber, or burn spill residue on sand and soda ash absorbent in a furnace.
Properties of anisidine
| EPA Substance Registry System | Anisidine (29191-52-4) |
Safety information for anisidine
Computed Descriptors for anisidine
New Products
8-Bromoisoquinoline 3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 1-Benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-ylidene)methyl]piperidine 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Trans-methyl 4-aminocyclohexane- carboxylate HCl 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE Xanomeline Suzetrigine 5-(5,5-Dimethyl-1,3,2-dioxaborinan-2-yl)-3-methyl-1,3-benzoxazol-2(3H)-one Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride Remibrutinib 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Variamine Blue B Diazonium salt Lead II Bromide N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Zinc Chloride Solution (All Grades) Calcium Alphaketoglutarate* L-Glycine methyl ester.HCl H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-L-Glu(OtBu)-OH.H2OYou may like
-
 2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
111771-08-5 -
![3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
2304754-51-4 -
 2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
1073435-67-2 -
 1589503-95-6 98+View Details
1589503-95-6 98+View Details
1589503-95-6 -
 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2477812-43-2 -
 Phenylazomalononitrile 98+View Details
Phenylazomalononitrile 98+View Details
6017-21-6 -
 KT-474 98+View Details
KT-474 98+View Details
2432994-31-3 -
 7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7
