ANGIOTENSIN I/II (1-6)
- CAS NO.:47896-63-9
- Empirical Formula: C36H55N11O10
- Molecular Weight: 801.89
- MDL number: MFCD00214589
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
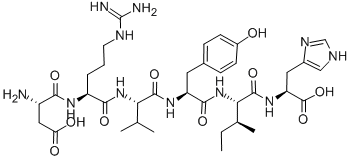
What is ANGIOTENSIN I/II (1-6)?
Biological Activity
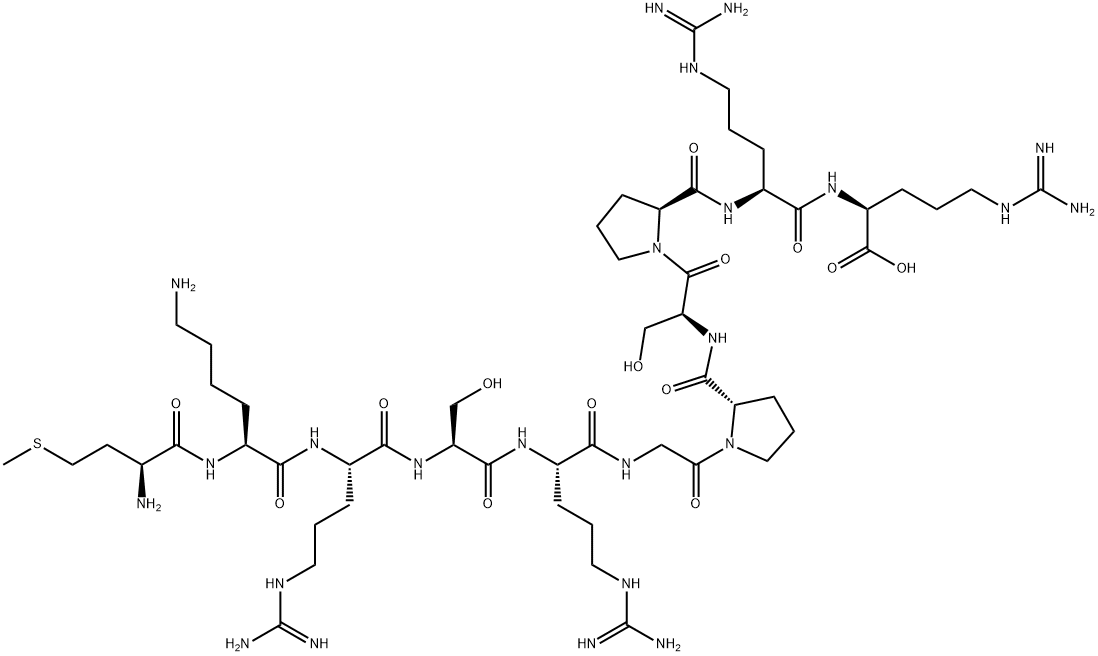
angiotensin i/ii (1-6) is a peptide (asp-arg-val-tyr-ile-his) that contains the amino acids 1-6 and is converted from angiotensin i/ii peptide.angiotensin i is formed by the action of renin on angiotensinogen, an α-2-globulin with 12 amino acids. angiotensinogen is produced constitutively and released into the circulation mainly by the liver. renin cleaves the peptide bond between the leucine (leu) and valine (val) residues on angiotensinogen, creating the ten-amino acid peptide angiotensin i. angiotensin i is converted to angiotensin ii (aii) through removal of two c-terminal residues by angiotensin-converting enzyme (ace), primarily by ace within the lung.angiotensin is a peptide hormone that causes vasoconstriction and a subsequent increase in blood pressure. angiotensin also stimulates the release of aldosterone, which promotes sodium retention in the distal nephron, thereby increasing blood pressure.figure1 formula of angiotensin i/ii (1-6)
References
1. Basso N, Terragno NA (December 2001). "History about the discovery of the renin-angiotensin system". Hypertension 38 (6): 1246–9. 2. Richard A. Preston. et. (1998). “Age-Race Subgroup Compared With Renin Profile as Predictors of Blood Pressure Response to Antihypertensive Therapy”. JAMA. 1998;280(13):1168-1172.3. Williams GH, Dluhy RG (2008). "Chapter 336: Disorders of the Adrenal Cortex". In Loscalzo J, Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL. Harrison's principles of internal medicine. McGraw-Hill Medical.
Properties of ANGIOTENSIN I/II (1-6)
| storage temp. | -15°C |
| solubility | insoluble in EtOH; ≥62.4 mg/mL in H2O; ≥80.2 mg/mL in DMSO |
| form | solid |
Safety information for ANGIOTENSIN I/II (1-6)
Computed Descriptors for ANGIOTENSIN I/II (1-6)
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1