ANGELIC ACID
- CAS NO.:565-63-9
- Empirical Formula: C5H8O2
- Molecular Weight: 100.12
- MDL number: MFCD00002654
- EINECS: 209-284-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
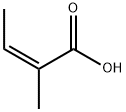
What is ANGELIC ACID?
Description
Angelic acid (cis-2-methyl-2-butenoic acid) is an unsaturated aliphatic carboxylic acid that was discovered by Munich pharmacist Ludwig Andreas Buchner in 1842. Buchner isolated it from the carrot-like roots of the herb Angelica archangelica (garden angelica). According to lore, an archangel revealed the herb’s medicinal properties, hence the heavenly name.
Since its discovery, angelic acid and its esters have been found in other plants, such as Roman chamomile (Anthemis nobilis), sabadilla (Schoenocaulon officinale), and lovage (Levisticum officinale). It is often found together with its more thermodynamically stable trans isomer, tiglic acid1.
In 1949, Robert E. Buckles* and Gene V. Mock at Iowa State University (Ames) reported the synthesis of tiglic acid by treating 2-hydroxy-2-methylbutyronitrile2 with sulfuric acid, followed by hydrolysis. They then converted tiglic acid to angelic acid in a three-step process: (1) addition of bromine across the double bond; (2) reaction with potassium hydroxide to form 3-bromoangelic acid3; and (3) reduction with sodium amalgam to produce angelic acid. Six years later, Buckles, Mock, and Louis Locatell, Jr., wrote an extensive review of the chemistry of both isomers.
Angelic and tiglic acids are used primarily as their esters in such products as perfumes and flavorings. The angelic ester petasin4, originally discovered in the common butterbur plant (Petasites hybridus), is a sesquiterpene with potential medical uses.
Despite its name, angelic acid has a spicy—even pungent—odor and a biting, acidic taste. As shown in the hazard information table, it must be handled with care.
1. CAS Reg. No. 80-59-1.
2. CAS Reg. No. 4111-08-4.
3. CAS Reg. No. 35057-99-9.
4. CAS Reg. No. 26577-85-5.
Chemical properties
White to light yellow crystal-powder
The Uses of ANGELIC ACID
Flavoring extracts.
Definition
ChEBI: The (Z)-isomer of 2-methylbut-2-enoic acid. It is found in plant species of the family Apiaceae.
Synthesis
Among many methods know n, one uses 2-Hydroxy-2-buty ronitrile to produce Tiglic acid, from which Angelic can be made by Bromine treatment. Another method goes directly from alpha-H ydroxy-alphu-me~h ylbutyric acid which by dehydration yields Angelic acid, or a mixture of Angelic and Tiglic acids.
Properties of ANGELIC ACID
| Melting point: | 44°C |
| Boiling point: | 96 °C / 12mmHg |
| Density | 1.010 |
| refractive index | nD47 1.4434 |
| Flash point: | 96℃ |
| storage temp. | Sealed in dry,2-8°C |
| solubility | almost transparency in Methanol |
| form | powder to crystal |
| appearance | white crystals or powder or colorless liquid |
| pka | pK (25°) 4.30 |
| color | White to Light yellow |
| Odor | at 100.00 %. spicy |
| Merck | 14,646 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 565-63-9(CAS DataBase Reference) |
Safety information for ANGELIC ACID
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P390:Absorb spillage to prevent material damage. P405:Store locked up. P406:Store in corrosive resistant/… container with a resistant inner liner. P501:Dispose of contents/container to..… |
Computed Descriptors for ANGELIC ACID
| InChIKey | UIERETOOQGIECD-ARJAWSKDSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 565-63-9 ANGELIC ACID 98%View Details
565-63-9 ANGELIC ACID 98%View Details
565-63-9 -
 Angelic acid 97% CAS 565-63-9View Details
Angelic acid 97% CAS 565-63-9View Details
565-63-9 -
 Angelic Acid CAS 565-63-9View Details
Angelic Acid CAS 565-63-9View Details
565-63-9 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
