Amphomycin
- CAS NO.:1402-82-0
- Empirical Formula: C58H91N13O20
- Molecular Weight: 1290.43
- MDL number: MFCD00864954
- EINECS: 215-760-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
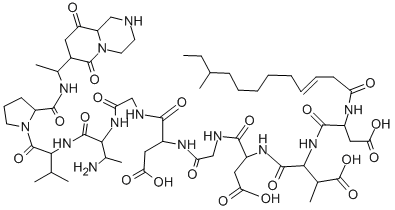
What is Amphomycin?
Originator
Amphocortrin CR,Warner Lambert,US,1963
The Uses of Amphomycin
Amphomycin is a lipopeptide antibiotic produced by Streptomycetes and Actinoplanes, initially reported by researchers at Bristol-Myers in 1953 from Streptomyces canus. Amphomycin was marketed as a complex of closely related analogues in the 1950s and 1960s. Structure elucidation was not completed until 2000. Amphomycin is active against Gram positive bacteria, inhibiting peptidoglycan synthesis and blocking cell wall development. Amphomycin is closely related to a number of “lost” antibiotics, aspartocin, crystallomycin, glumamycin, friulimicin, laspartocin, tsushimycin and zaomycin. Interest in amphomycin was re-awakened with the discovery of friulimicin activity against antibiotic resistant strains.
Manufacturing Process
The process for producing amphomycin comprises cultivating a strain of
Streptomyces canus in an aqueous, nutrient-containing carbohydrate solution
under submerged aerobic conditions until substantial antibacterial activity is
imparted to the solution and then recovering the so-produced amphomycin
from the fermentation broth.
The process of decolorizing solutions of amphomycin then involves treatment
with activated charcoal, followed by the steps of (1) extracting the antibiotic
into a water-immiscible organic solvent under strongly acid conditions or
precipitating the amphomycin from aqueous solution by adjusting the pH to a
point within the range of pH 3.0 to 4.0, (2) removing impurities from strongly
acid, aqueous solution of amphomycin by extraction of the impurities with
methyl isobutyl ketone and amyl acetate, (3) extracting the amphomycin from
a strongly acid solution in butanol by the use of water having a pH higher
than 4, (4) extracting the amphomycin from solution in water-immiscible
organic solvent into water whose pH is greater than 6.0, (5) precipitating
amphomycin from solution by formation of insoluble derivatives of the basic
function, and (6) precipitating amphomycin from solution by formation of
insoluble derivates of the acidic function.
The amphomycin is then converted to the calcium salt with calcium hydroxide.
Therapeutic Function
Antibiotic
Biological Activity
amphomycin is a natural antibacterial lipopeptide.cyclic lipopeptides are a promising class of natural products with antibiotic properties. cyclic lipopeptides are amphiphilic molecules, composed of a fatty acid tail linked to a short oligopeptide which form a macrocylic ring structure.
Safety Profile
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion.Induces hemolysis. Active against gram-positive bacteria.Suggested as a topical agent for animal and plantinfections. When heated to decomposition it emits acridsmoke and
in vitro
in previous study, calf brain endoplasmic reticulum membranes were incubated with varying concentrations of gdp-mannose in the presence and absence of amphomycin, results showed no significant difference in apparent km for gdp-mannose. however, the vmax was reduced in the presence of amphomycin as compared with in its absence. moreover, when mannosylphosphoryldolichol synthase activity was measured in the presence of amphomycin, the shape of the substrate velocity curve changed from a rectangular hyperbola to a sigmoid [1].
in vivo
the pk of lipopeptides, the semi-synthetic amphomycin analogues, were evaluated in mice and rats following single i.v. and oral administration. following oral administration at 50 mg/kg, plasma concentrations of amphomycin analogues were
References
[1] d. k. banerjee. amphomycin inhibits mannosylphosphoryldolichol synthesis by forming a complex with dolichylmonophosphate. the journal of biological chemisty 264(4), 2024-2028 (1989).
[2] pasetka cj, erfle dj, cameron dr, clement jj, rubinchik e. novel antimicrobial lipopeptides with long in vivo half-lives. int j antimicrob agents. 2010 feb;35(2):182-5.
Properties of Amphomycin
| Boiling point: | 854.06°C (rough estimate) |
| alpha | D25 +7.5° (c = 1 at pH 6) |
| Density | 1.0985 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| color | Crystals |
Safety information for Amphomycin
Computed Descriptors for Amphomycin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
