Ammonium Sulfite
- CAS NO.:10196-04-0
- Empirical Formula: H8N2O3S
- Molecular Weight: 116.14
- MDL number: MFCD00066094
- EINECS: 233-484-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
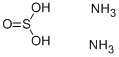
What is Ammonium Sulfite?
Chemical properties
Ammonium sulfite is a colorless to yellow crystalline (sand-like or sugar-like) solid, normally sold or used in a 40% solution. It is used both as a reducing agent and in photography. This compound is a salt that can form from the reaction of ammonia and sulfur dioxide or hydrogen sulfide. This product is frequently found in the overhead of crude distillation units and can be quite corrosive to steel equipment. Chemical corrosion inhibitors are often used to control corrosion from ammonium sulfite and other ammonium salts.
Physical properties
Colorless monoclinic crystals. Soluble in water, its aqueous solution is alkaline. Slightly soluble in alcohol. Insoluble in acetone and carbon disulfide. It is easily oxidized to ammonium sulfate in air. It can be decomposed into ammonia and sulfur dioxide when heated.
The Uses of Ammonium Sulfite
Ammonium Sulfite is an additive used in the production of caramel.
Preparation
In the ATS process, sulfur dioxide is absorbed from incinerated Claus tail gas in aqueous ammonia to produce ammonium sulfite and ammonium bisulfite in solution.
Definition
ChEBI: Diammonium sulfite is a p-block molecular entity and a sulfite salt.
General Description
AMMONIUM SULFITE is a colorless crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. AMMONIUM SULFITE is used in the manufacture of other chemicals, in medicine, and photography.
Air & Water Reactions
Soluble in water.
Reactivity Profile
AMMONIUM SULFITE is a reducing agent. Emits toxic sulfur dioxide and oxides of nitrogen when heated to decomposition [USCG, 1999].
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes.
Fire Hazard
Special Hazards of Combustion Products: Toxic sulfur dioxide and oxides of nitrogen may form in fires.
Potential Exposure
Ammonium sulfite is used in medicines, metal lubricants; explosives, photography, hair wave solutions; and to make other chemicals. It is also used as a preservative, and for treating agricultural grain.
Shipping
UN2693 Bisulfites, inorganic, aqueous solutions, n.o.s., Hazard class: 8; Labels: 8-Corrosive material. UN3077 Environmentally hazardous substances, solid, n.o. s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
A strong reducing agent. Reacts violently with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,state, and local environmental regulations must be observed. May be buried in a chemical waste landfill in accordance with federal, state, and local statutes; or, if oxidized and neutralized, it may be sent to a municipal sewage treatment plant for biological treatment Incineration.
Properties of Ammonium Sulfite
| Melting point: | 60°C |
| Density | 1.41(25℃) |
| form | solid (rough estimate) |
| Water Solubility | 642g/L at 25℃ |
| Stability: | Stable. Efflorescent. Incompatible with acids, strong oxidizing agents. |
| CAS DataBase Reference | 10196-04-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium sulfite (10196-04-0) |
Safety information for Ammonium Sulfite
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium Sulfite
Related products of tetrahydrofuran





You may like
-
 10196-04-0 / 17026-44-7 Ammonium sulfite 98%View Details
10196-04-0 / 17026-44-7 Ammonium sulfite 98%View Details
10196-04-0 / 17026-44-7 -
 Ammonium sulfite solution CAS 10196-04-0View Details
Ammonium sulfite solution CAS 10196-04-0View Details
10196-04-0 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3