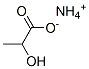
Ammonium Lactate
- CAS NO.:515-98-0
- Empirical Formula: C3H9NO3
- Molecular Weight: 107.11
- MDL number: MFCD00036411
- EINECS: 208-214-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is Ammonium Lactate?
Absorption
In vitro study of percutaneous absorption of ammonium lactate lotion, 12% using human cadaver skin indicates that approximately 5.8% of the material was absorbed after 68 hours.
Toxicity
The oral administration of Lac-Hydrin to rats and mice showed this drug to be practically non-toxic (LD50 > 15 mL/kg).
Description
Ammonium lactate is a compound with formula NH4(C2H4(OH)COO). It is the ammonium salt of lactic acid.
It has E number "E328" and is the active ingredient of the skin lotions Amlactin and Lac-Hydrin.
The Uses of Ammonium Lactate
When ammonium lactate is applied topically, it is found to thicken the viable epidermis while reducing the thickness of the stratum corneum layer. It is a neutralized version of lactic acid.
The Uses of Ammonium Lactate
Antipruritic (topical).
The Uses of Ammonium Lactate
Ammonium lactate solution has been used in the ammonium lactate fermentation broth for the concentration of organic acid salts by conventional electrodialysis (EDC).
Indications
For the treatment of dry, scaly skin (xerosis) and ichthyosis vulgaris and for temporary relief of itching associated with these conditions.
Background
Ammonium lactate is the ammonium salt of lactic acid.
brand name
Lac-Hydrin (Westwood-Squibb).
General Description
Ammonium lactate is an ammonium salt of lactic acid. It is generally used as a feedstock for ethyl lactate and as a component of moisturizers.
Industrial uses
Ammonium lactate, which is produced in a fermentation process, is thermally and catalytically cracked to produce lactic acid, which upon addition of an alcohol generates the ester. The ammonia and water byproducts are separated through a selective membrane and recycled. This process, which uses carbohydrate feedstocks, has made the production of lactate esters economically competitive. In turn, due to the excellent solvent properties of ethyl lactate, it has become widely available as a bio-sourced and biodegradable cleaning fluid.It has also found industrial applications in speciality coatings and inks. Archer Daniels Midland (ADM), an agricultural processing company who have been commercializing the production of ethyl lactate, have recently patented isoamyl lactate as a component in an environmentally friendly solvent and household cleaner.
Pharmacokinetics
Lactic acid is an alpha-hydroxy acid. It is a normal constituent of tissues and blood. The alpha-hydroxy acids (and their salts) may act as humectants when applied to the skin. This property may influence hydration of the stratum corneum. In addition, lactic acid, when applied to the skin, may act to decrease corneocyte cohesion.
Metabolism
Not Available
Properties of Ammonium Lactate
| Melting point: | 91-94 °C |
| Boiling point: | 74 °C(Press: 0.14 Torr) |
| Density | 1.054 g/mL at 25 °C |
| refractive index | n |
| storage temp. | Store at -20°C |
| form | Liquid |
| color | Colorless to light yellow |
| PH | 6.58(1 mM solution);6.56(10 mM solution);6.55(100 mM solution);6.5(1000 mM solution) |
| Water Solubility | Water: 125 mg/mL (1167.02 mM) |
| CAS DataBase Reference | 515-98-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium lactate (515-98-0) |
Safety information for Ammonium Lactate
Computed Descriptors for Ammonium Lactate
Ammonium Lactate manufacturer
HRV Global Life Sciences
ShanPar Industries Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 515-98-0 / 52003-58-4 Ammonium lactate 99%View Details
515-98-0 / 52003-58-4 Ammonium lactate 99%View Details
515-98-0 / 52003-58-4 -
 515-98-0 / 52003-58-4 99%View Details
515-98-0 / 52003-58-4 99%View Details
515-98-0 / 52003-58-4 -
 Ammonium lactate 98%View Details
Ammonium lactate 98%View Details
515-98-0 / 52003-58-4 -
 Ammonium lactate solution CAS 515-98-0View Details
Ammonium lactate solution CAS 515-98-0View Details
515-98-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1