AMMONIUM CHROMATE
Synonym(s):Chromic acid ammonium salt
- CAS NO.:7788-98-9
- Empirical Formula: CrH8N2O4
- Molecular Weight: 152.07
- MDL number: MFCD00015958
- EINECS: 232-138-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
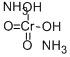
What is AMMONIUM CHROMATE?
Chemical properties
Ammonium chromate is a yellow crystalline compound which can be used in solution, which is yellow with an ammonia odor.
The Uses of AMMONIUM CHROMATE
Ammonium chromate, is used in photography as a sensitizer for gelatin coatings. It's often used in photography, textile printing, and fixing chromate dyes on wool. It is also used as an analytical reagent, catalyst.
The Uses of AMMONIUM CHROMATE
Sensitizing gelatin in photography, in textile printing pastes, in fixing chromate dyes on wool, and as a reagent in analytical chemistry.
The Uses of AMMONIUM CHROMATE
Reagent for the preparation of a solid heterogeneous oxidizing agent for liquid phase oxidation of alcohols.
Hazard
Toxic by inhalation, strong irritant. Con- firmed carcinogen.
Safety Profile
A poison. Mutation data reported. See also CHROMIUM COMPOUNDS. A powerful oxidizer. An explosion hazard when shocked or heated. When heated to decomposition it emits toxic fumes of NH3, Cr03, and NOx. Incompatible with reducing agents.
Potential Exposure
It is used to inhibit corrosion and in dyeing, photography and many chemical reactions. Used as a fungicide and fire retardant.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it from weak aqueous ammonia (ca 2.5mL/g) by cooling from room temperature. It loses NH3 on heating to form ammonium dichromate. POISONOUS.
Incompatibilities
A strong oxidizer and an explosive. Contact with combustible, organic and other readily oxidizable substances may cause fire and explosions. Hydrazine, other reducing agents. Corrosive to metals.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Addition of a large volume of a reducing agent solution (hypo, bisulfite or ferrous salt, and acidify with 3 M sulfuric acid). When reduction is complete, flush to drain with large volumes of water.
Properties of AMMONIUM CHROMATE
| Melting point: | 180°C (dec.) |
| Density | 1.91 g/mL at 25 °C (lit.) |
| solubility | H2O: 1 M at 20 °C, clear, yellow |
| form | Crystalline |
| Specific Gravity | 1.91 |
| color | Yellow-orange |
| Water Solubility | Soluble in water. Slightly soluble in liquid ammonia, acetone, methanol. Insoluble in ethanol. |
| Merck | 14,510 |
| Exposure limits | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| CAS DataBase Reference | 7788-98-9(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium chromate (7788-98-9) |
Safety information for AMMONIUM CHROMATE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H314:Skin corrosion/irritation H334:Sensitisation, respiratory H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AMMONIUM CHROMATE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 7788-98-9 AMMONIUM CHROMATE 99%View Details
7788-98-9 AMMONIUM CHROMATE 99%View Details
7788-98-9 -
 Ammonium chromate CAS 7788-98-9View Details
Ammonium chromate CAS 7788-98-9View Details
7788-98-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
