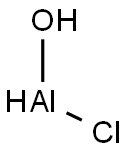
Aminomercuric chloride
Synonym(s):Amidomercury(II) chloride
- CAS NO.:10124-48-8
- Empirical Formula: ClH2HgN
- Molecular Weight: 252.07
- MDL number: MFCD00016140
- EINECS: 233-335-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-15 16:24:08
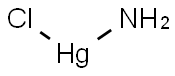
What is Aminomercuric chloride?
Chemical properties
white powder
The Uses of Aminomercuric chloride
Anti-infective, topical.
The Uses of Aminomercuric chloride
Mercury ammonium chloride is an inorganic mercurial compound used in creams as a topical anti-infective agent (formerly used in the treatment of psoriasis and in skin-lightening formulations).
General Description
A white crystalline solid. Corrosive to the mucous membranes. Very toxic by inhalation and ingestion.
Air & Water Reactions
Slightly water soluble. Decomposes in hot water.
Reactivity Profile
Aminomercuric chloride is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper .
Health Hazard
The general symptoms are those of mercury poisoning, developing rapidly after ingestion but more slowly after low repeated exposures. Contact with eyes causes irritation and ulceration. Skin contact may cause dermatitis. Ingestion causes pain, vomiting, metallic taste, ulceration of mouth and stomach, pallor, and rapid, weak pulse.
Fire Hazard
Special Hazards of Combustion Products: Smoke may contain toxic mercury compounds.
Safety Profile
A poison by ingestion. Moderately toxic by skin contact. Explosive reaction with halogens or amine metal salts. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and Hg. See also MERCURY COMPOUNDS.
Properties of Aminomercuric chloride
| Melting point: | 383°C (dec.) |
| Boiling point: | sublimes [CRC10] |
| Density | 5.38 |
| solubility | insoluble in H2O, ethanol; swarm acid solutions |
| form | white solid |
| color | White powder |
| Odor | Odorless |
| Water Solubility | insoluble H2O, EtOH; soluble warm acid [CRC10] |
| Merck | 13,5902 |
| Stability: | Stable. |
| CAS DataBase Reference | 10124-48-8(CAS DataBase Reference) |
| EPA Substance Registry System | Mercury amide chloride (Hg(NH2)Cl) (10124-48-8) |
Safety information for Aminomercuric chloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Aminomercuric chloride
| InChIKey | WRWRKDRWMURIBI-UHFFFAOYSA-M |
Abamectin manufacturer
Samuh Laxmi Chemicals (Bom) P Ltd
L S Chemicals and Pharmaceuticals
Pallav Chemicals And Solvents Pvt Ltd
ARRAKIS INDUSTRIES LLP
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)

You may like
-
 Ammonium mercuric chloride 98%View Details
Ammonium mercuric chloride 98%View Details
10124-48-8 -
 MERCURY (II) AMMONIUM CHLORIDE 10124-48-8 99%View Details
MERCURY (II) AMMONIUM CHLORIDE 10124-48-8 99%View Details
10124-48-8 -
 MERCURY AMMONIATE CAS 10124-48-8View Details
MERCURY AMMONIATE CAS 10124-48-8View Details
10124-48-8 -
 Mercuric ammonium chloride, ≥97% CAS 10124-48-8View Details
Mercuric ammonium chloride, ≥97% CAS 10124-48-8View Details
10124-48-8 -
 Mercury(II) amidochloride CAS 10124-48-8View Details
Mercury(II) amidochloride CAS 10124-48-8View Details
10124-48-8 -
 10124-48-8 99%View Details
10124-48-8 99%View Details
10124-48-8 -
 Mercury ammoniated 98.3% CAS 10124-48-8View Details
Mercury ammoniated 98.3% CAS 10124-48-8View Details
10124-48-8 -
 MERCURY AMMONIATED Extra Pure CAS 10124-48-8View Details
MERCURY AMMONIATED Extra Pure CAS 10124-48-8View Details
10124-48-8