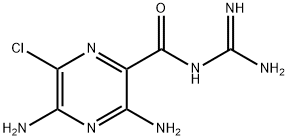
AMILORIDE
- CAS NO.:2609-46-3
- Empirical Formula: C6H8ClN7O
- Molecular Weight: 229.63
- MDL number: MFCD00077316
- EINECS: 220-024-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:16
What is AMILORIDE?
Absorption
Readily absorbed following oral administration.
Toxicity
No data are available in regard to overdosage in humans. The oral LD50 of amiloride hydrochloride (calculated as the base) is 56 mg/kg in mice and 36 to 85 mg/kg in rats, depending on the strain. The most likely signs and symptoms to be expected with overdosage are dehydration and electrolyte imbalance.
Description
Amyloride is also a potassium sparing diuretic that exhibits moderate activity. It is not an antagonist of aldosterone. It inhibits reabsorption of sodium ions and reduces excretion of potassium ions.
Originator
Midamor,Merck,UK,1971
The Uses of AMILORIDE
Amiloride is an epithelial sodium channel blocker.
The Uses of AMILORIDE
Diuretic.
The Uses of AMILORIDE
Amyloride is rarely used individually—as a rule it is used in combination with thiazides or loop diuretics. It is mainly used in combination with thiazide diuretics for cardiac insufficiency and hypertension, especially in cases where it is necessary to prevent hypokalemia.
Background
A pyrazine compound inhibiting sodium reabsorption through sodium channels in renal epithelial cells. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with diuretics to spare potassium loss. (From Gilman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th ed, p705)
Indications
For use as adjunctive treatment with thiazide diuretics or other kaliuretic-diuretic agents in congestive heart failure or hypertension.
Definition
ChEBI: A monocarboxylic acid amide that is N-carbamimidoylpyrazine-2-carboxamide substituted by amino groups at positions 3 and 5 and a chloro group at position 6.
Manufacturing Process
Step A: Preparation of methyl 3-amino-5,6-dichloropyrazinoare: Methyl 3-
aminopyrazinoate (765 g, 5 mols) is suspended in 5 liters of dry benzene.
While stirring under anhydrous conditions sulfuryl chloride (1.99 liters, 3.318
g, 24.58 mols) is added over a period of 30 minutes and stirring is continued
for 1 hour. During this period, the temperature rises to about 50°C and then
begins to drop. The mixture is heated cautiously to reflux (60°C), refluxed for
5 hours and then stirred overnight at room temperature. The excess sulfuryl
chloride is distilled off at atmospheric pressure (distillation is stopped when
vapor temperature reaches 78%). The dark red mixture is chilled to 6°C. The
crystals are filtered off, washed by displacement with two 100 ml portions of
cold (8°C) benzene, then washed with 300 ml petroleum ether and dried in
vacuum at room temperature, yielding 888 g (80%) of methyl 3-amino-5,6-
dichloropyrazinoate in the form of red crystals, MP 228-230°C. The crude
product is dissolved in 56 liters of boiling acetonitrile and passed through a
heated (70-80°C) column of decolorizing charcoal (444 g). The column is
washed with 25 liters of hot acetonitrile, the combined eluate concentrated in
vacuum to about 6 liters and chilled to 5°C. The crystals that form are
filtered, washed three times with cold acetonitrile, and air dried to constant
weight, yielding 724 g (82% recovery, 66% overall) of methyl 3-amino-5,6-
dichloropyrazinoate in the form of yellow crystals, MP 230-234°C. After
additional recrystallizations from acetonitrile the product melts at 233-234°C.
Step B: Preparation of methyl-3,5diamino-6-chloropyrazinoete: In a 2-liter, 3-necked flask fitted with a a mechanical stirrer, thermometer and gas inlet tube
is placed dry dimethyl sulfoxide (1 liter). Methyl 3-amino-5,6-
dichloropyrazinoate (100 g, 0.45 mol) is added and the mixture stirred and
heated at 65°C on a steam bath until solution is effected. A stream of dry
ammonia gas is admitted to the solution with continuous stirring, over a
period of 45 minutes while the temperature is maintained at 65-70°C. The
solution is cooled to about 10°C with continuous stirring and ammonia gas is
admitted for an additional 1 1/4 hours. The yellow reaction mixture is poured,
with stirring, into cold water (2 liters) and the light yellow solid that separates
is removed by filtration, thoroughly washed with water, and dried in a vacuum
desiccator to give 82.5 g (91%) of methyl 3,5-diamino-6-chloropyrazinoate,
MP 210-212°C. Recrystallization from acetonitrile gives material melting at
212-213°C.
Step C: Preparation of the base: A 300 ml one-necked, round-bottomed flask,
equipped with a water-cooled condenser, calcium chloride tube and magnetic
stirrer is charged with anhydrous methanol (150 ml) and sodium metal (5.75
g, 0.25 g atom). When the reaction is complete, the solution is treated with
dry guanidine hydrochloride (26.3 g, 0.275 mol) and stirred for 10 minutes.
The sodium chloride that forms is removed by filtration. The solution is
concentrated in vacuum to a volume of 30 ml and the residue treated with the
product of Step B, heated one minute on a steam bath and kept at 25°C for 1
hour. The product is filtered, washed well with water, dissolved in dilute
hydrochloric acid and the free base precipitated by addition of sodium
hydroxide to give the amiloride product base, a solid which melts at 240.5-
241.5°C.
To produce the hydrochloride, the base is suspended in water (70 ml) and
treated with sufficient 6 N hydrochloric acid to dissolve the free base. The
solution is filtered and treated with concentrated hydrochloric acid (5 ml). The
hydrochloride salt (22 g, 97%) separates and is recrystallized from water (50
ml) containing concentrated hydrochloric acid (3 ml).
brand name
Midamor (Merck).
Therapeutic Function
Diuretic
Pharmacokinetics
Amiloride, an antikaliuretic-diuretic agent, is a pyrazine-carbonyl-guanidine that is unrelated chemically to other known antikaliuretic or diuretic agents. It is an antihypertensive, potassium-sparing diuretic that was first approved for use in 1967 and helps to treat hypertension and congestive heart failure. The drug is often used in conjunction with thiazide or loop diuretics. Due to its potassium-sparing capacities, hyperkalemia (high blood potassium levels) are occasionally observed in patients taking amiloride. The risk is high in concurrent use of ACE inhibitors or spironolactone. Patients are also advised not to use potassium-containing salt replacements.
Clinical Use
Amiloride, another potassium-sparing diuretic, is an aminopyrazine structurally related to triamterene as an open-chain analogue. Similar to triamterene, it interferes with the process of cationic exchange in the distal convoluted tubule by blocking luminal sodium channels. It blocks the reabsorption of sodium ion and the secretion of potassium ion. It has no effect on the action of aldosterone. Oral amiloride is approximately 50% absorbed, with a duration of action of 10 to 12 hours, which is slightly longer than that for triamterene. Although triamterene is extensively metabolized, approximately 50% of amiloride is excreted unchanged. Renal impairment can increase its elimination half-life. Like triamterene, amiloride combined with a thiazide or loop diuretic is used to treat edema or hypertension.
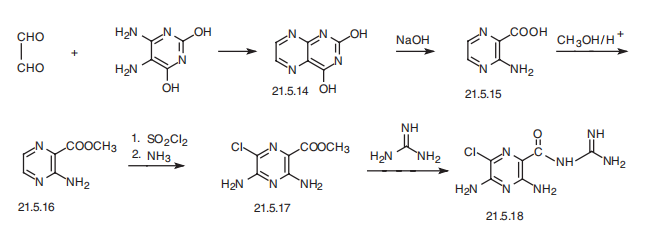
Synthesis
Amyloride, N-amidino-3,5-diamino-6-chloropirazincarboxamide (21.5.18), is synthesized from 5,6-diaminouracil, which upon reaction with glyoxal transforms into a pyrazineopyrimidine derivative (21.5.14), which decomposes upon further reaction with a strong alkaline, forming 3-aminopirazin-2-carboxylic acid (21.5.15). This is esterified into the corresponding methyl ester (21.5.16), and subsequently treated with sulfonyl chloride and ammonia, which gives the methyl ester of 3,5-diamino-6-chloropirazin-2-carboxylic acid (21.5.17). Reacting this with guanidine gives amyloride (21.5.18).
Enzyme inhibitor
This potassium-sparing diuretic (FW = 229.63 g/mol; CAS 2016-88-8; Absorbance at 10 mg/mL (water) = 642 at 212 nm, 555 at 285 nm, and 617 at 362 nm; pKa = 8.7; Soluble in hot water (50 mg/mL), yielding a clear, yellow-green solution), also known as MK 870, Midamor?, and 3,5- diamino-6-chloro-N- (diaminomethylene) pyrazine-2-carboxamide, is an epithelial sodium channel (or ENaC) blocker used to manage hypertension and congestive heart failure (1-4). Two classes of Na+ transporters are sensitive to this drug: (a) the conductive Na+ entry pathway found in electrically high resistance epithelia and (b) a Na+-H+ electroneutral exchange system found in certain leaky epithelia, such as the renal proximal tubule. Amiloride exhibits a Ki value of <1 μM for the kidney transporter and approximately 1 mM in the colon. Midamor is a potassium- conserving (antikaliuretic) drug that possesses weak (compared with thiazide diuretics) natriuretic, diuretic, and antihypertensive activity. Like other potassium-conserving agents, amiloride may cause hyperkalemia (i.e., serum K+ levels >5.5 mEq/L), which, if uncorrected, can be fatal. Amiloride-induced hyperkalemia occurs in ~10% of patients, when used without a kaliuretic diuretic. This complication is more frequent in patients showing evidence of renal impairment and diabetes mellitus. When used along with a thiazide diuretic in patients without these complications, the risk of Amiloride-induced hyperkalemia drops to 1-2%. Amiloride is not metabolized by the liver and is instead excreted unchanged by the kidney. Amiloride displaces both adenosine A1 receptor agonist and antagonist binding with a Ki value in the low micromolar range, when assayed in calf brain membranes. Inhibition is counteracted by NaCl and protons. Amiloride (IC50 = 0.25 mM) and 5- (N-ethyl-N-isopropyl) amiloride (IC50 = 0.11 mM) also inhibit coxsackievirus B3 RNA polymerase in a single- nucleotide incorporation assay, although the argument that amiloride competes with incoming nucleoside triphosphates is unconvincing. A high- fidelity RNA dependent CVB3 RNA polymerase (RdRp) variant is amiloride-resistant.
Metabolism
Amiloride is not metabolized by the liver but is excreted unchanged by the kidneys.
Properties of AMILORIDE
| Melting point: | 240.5-241.5° |
| Density | 2.11±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | pKa 8.7 (Uncertain) |
| form | Solid |
| color | Off-white to light yellow |
| Water Solubility | 659g/L(25 ºC) |
| CAS DataBase Reference | 2609-46-3(CAS DataBase Reference) |
Safety information for AMILORIDE
Computed Descriptors for AMILORIDE
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran



You may like
-
 Amiloride 98%View Details
Amiloride 98%View Details -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0