Amfonelic acid
Synonym(s):7-Benzyl-1-ethyl-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid
- CAS NO.:15180-02-6
- Empirical Formula: C18H16N2O3
- Molecular Weight: 308.33
- MDL number: MFCD00055095
- EINECS: 605-001-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
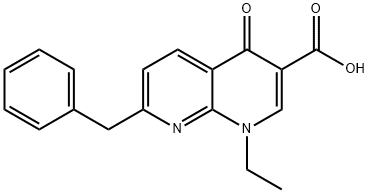
What is Amfonelic acid?
Description
Amfonelic acid (AFA) is a dopamine reuptake inhibitor. Experiments on rats have shown that AFA treatment completely prevented the effects of methamphetamine on the dopaminergic system, both morphologically and biochemically.
Description
Amfonelic acid, a derivative of nalidixic acid, was originally developed as a central nervous system stimulant. It is a highly potent nonamphetamine stimulant whose actions are inhibited by reserpine but not by the tyrosine hydroxylase inhibitor, a-methyltyrosine. Additionally, it enhances the impacts of antipsychotic medicines such as haloperidol, trifluoperazine, and spiperone.
The Uses of Amfonelic acid
Stimulant (central).
Biological Activity
Amfonelic acid (AFA), an inhibitor of dopamine (DA) uptake, has been found to increase the levels of tryptophan (TRYP) and 5-hydroxyindoleacetic acid (5-HIAA) in the rat c. striatum, cerebral cortex, and brain stem. The duration of action of the effect of AFA on TRYP and 5-HIAA appeared to be longer than that of the increase of the striatal DA metabolites homovanillic acid and 3,4-dihydroxyphenylacetic acid[1].
References
The effects of amfonelic acid alone and in combination with naloxone on brain-stimulation reward DOI: 10.1016/0091-3057(89)90069-5
Pharmacologic properties and mechanism of action of amfonelic acid DOI: 10.1016/0014-2999(70)90206-2
References
[1] P C Waldmeier. “The effects of amfonelic acid on 5-HT metabolism in rat brain.” Journal of Neural Transmission 57 3 (1983): 149–65.
Properties of Amfonelic acid
| Melting point: | >1000 °C |
| Boiling point: | 499℃ |
| Density | 1.297 |
| Flash point: | 256℃ |
| solubility | 0.1 M NaOH: soluble |
| form | powder |
| pka | 3.50±0.20(Predicted) |
| color | yellow |
| InChI | InChI=1S/C18H16N2O3/c1-2-20-11-15(18(22)23)16(21)14-9-8-13(19-17(14)20)10-12-6-4-3-5-7-12/h3-9,11H,2,10H2,1H3,(H,22,23) |
Safety information for Amfonelic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Amfonelic acid
| InChIKey | WHHHJDGNBVQNAU-UHFFFAOYSA-N |
| SMILES | N1(CC)C2C(=CC=C(CC3=CC=CC=C3)N=2)C(=O)C(C(O)=O)=C1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Amfonelic Acid >95% CAS 15180-02-6View Details
Amfonelic Acid >95% CAS 15180-02-6View Details
15180-02-6 -
 Amfonelic Acid CAS 15180-02-6View Details
Amfonelic Acid CAS 15180-02-6View Details
15180-02-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1